
- Culture and Varieties
- Soils and Fertility
- Irrigation
- Sprayers
- Diseases
- Insect Management
- Weed Control
- Food Safety and Sanitation
- Harvest/Post-Harvest and Waste Management
- Marketing
- Production Costs
- Organic Production
Culture and Varieties
George Boyhan, Extension Horticulturist - Vegetables
Beans, including snap beans (Phaseolus vulgaris) and lima beans (P. lunatus), come in two basic types: bush and runner. Bush beans form a low-growing compact plant that stands 1 to 2 feet tall. Breeding has selected for short internodes in bush types so that the plant never gets very tall. These plants tend to set all of their pods at one time, facilitating a once-over harvest. The concentrated pod set is particularly well suited to machine harvesting. Runner types, which are often referred to as pole beans, form vines that require trellising. This type of plant will climb up trellises to form a larger plant with longer vines and reach about 6 to 7 feet tall. Runner types flower and set pods over a longer period of time. These plants are particularly well suited to pick-your-own and roadside marketers. A few varieties, known as half-runners, fall somewhere between a bush and runner type.
Lima beans are often referred to as butter beans, particularly in the South. The butter bean name refers to the significant difference in their nutritional content from snap beans. Lima beans have much higher amounts of protein, carbohydrates and calories than snap beans.
Most beans grown in Georgia are for the mature green market and are well sized for the variety but the seed in the pod remains immature. In the case of lima beans, the seeds have not become hard, although pods can be left on the plant to allow the seed to become hard and fully mature. Such seed would be shelled and used for the dry bean market or for seed. Unlike snap beans, lima beans are shelled before processing or preparing since the shell is tough, fibrous and inedible. There is not a commercially significant amount of dry bean production in Georgia. Most dry bean production is done in western states where drier conditions prevail.
Variety Selection
For processing, it is best to see if your buyer has specific recommendations or requirements for the crop before choosing a variety. Both bush and runner type snap bean pods can either be round (often called blue lake types) or flat (known as Kentucky wonder types).
Lima beans also have different bean sizes — baby limas and Fordhooks — that represent distinct sub-groups of this species. Baby limas are from Meso-America and are more heat tolerant, while the Fordhooks are from the cooler Andean region. This may be an important consideration when choosing varieties.
Table 1 lists different varieties for snap bean and lima bean production in Georgia. Snap beans are usually green in color, but yellow varieties called wax beans do exist. Wax beans have the same cultural requirements as snap beans. Wax beans are not grown widely for the commercial market in Georgia. Growers wishing to try new varieties should plant a small area to assess suitability for their situation.
Planting
Beans are always direct seeded as there is no advantage to transplanting. The seed are large and easily handled by appropriate seeding equipment. Commercial seed germination is excellent and very uniform. Seed is usually treated with an appropriate fungicide to prevent seedling diseases. Organic growers will have to source untreated or organically produced seed and should contact their seed company well in advance of planting to ensure availability. More and more, companies are specializing in organic seed or offering untreated seed in their catalogs.
Seeding equipment based on a number of different mechanisms is available for bean sowing. Whether the equipment uses plates, belts or vacuum to single out the seed, it should be calibrated to meet your required spacing and depth of planting. Between-row spacing is particularly critical for machine-harvested beans. Check to make sure this spacing is correct, especially if an outside company will be contracted to handle the harvest.
Spacing
Bush snap beans are typically sown with rows 30 to 36 inches apart. Snap beans are sown with 5 to 7 seed per linear foot of row, or with about 1.75 to 2.5 inches between each seed. Yields can be increased with rows planted 18 to 24 inches apart with 4 to 6 seed per linear foot of row (2 to 3 inches between seed). For runner types, the between-row spacing is 36 to 48 inches and the in-row spacing is 6 to 9 inches.
Bush lima beans are spaced 18 to 36 inches between-rows and 3 to 6 inches in the row. Runner or pole lima beans are spaced 36 to 48 inches between rows and 8 to 12 inches in the row. These spacings can be adjusted somewhat to facilitate specific field or equipment situations.
Bean seed are sown approximately 1 to 1.5 inches deep depending on the seed size and soil type. Generally, seed should be planted at a depth that is twice the diameter of the seed on its longest axis. Seed can be planted slightly deeper on light-textured soils like those found in the Coastal Plain. Seed depth can be shallower on heavier soils.
Trellising will be required for runner type beans. One method of trellising is to space 2x2 stakes approximately 15 to 20 feet apart and driven into the ground, leaving 5 to 6 feet above ground. Nail a 10- to 12-gauge wire to the top of the stake and another near the base about 5 to 6 inches above the ground. Loop twine around these wires in a cross-cross fashion. The bean vines will then grow up this structure.
The amount of seed required per acre can be determined based on row spacing, seed weight and germination rate. For bush snap beans, rates of 75 to 90 pounds per acre are required with typical plant spacings. For runner or pole snap beans, rates of 20 to 45 pounds per acre are required. Lima bean rates are 60 to 85 pounds per acre. Your seed company can supply you with the number of seed per ounce and the germination rate. Calculate the amount of seed to order based on this information and your planned spacing.
For example, with 103 seed per ounce, a germination rate of 94 percent and 2-foot in-row spacing and 36-inch between-row spacing, the plant population per acre is estimated at 87,120 and the amount of seed required per acre is 846 ounces, or 53 pounds. Finally, with a germination rate of 94 percent, a minimum of 56 pounds per acre would be required.
Temperature
Bean seed will germinate with a soil temperature of 60 to 85° F, with warmer temperatures resulting in faster germination. Seed germination with a soil temperature of 60° F can take up to two weeks, while at 85° F it can occur in seven days or less.
Cool soil temperatures and slow germination can lead to a higher incidence of seedling diseases. For this reason, growers using untreated seed should not plant into cool soils. In addition, soils prone to crusting can inhibit seedling emergence. This can be alleviated by keeping the soil surface moist, which allows seedlings to emerge easily. In south Georgia, recommended spring planting dates are from February 15 to April 30. Seedling emergence, however, will be slow with earlier sowing dates. For fall production in south Georgia, seed should be sown between July 15 and September 15. In north Georgia, recommended spring production sowing dates are May 1 to July 15; fall production is not recommended.
Beans grow optimally in a temperature range of 60 to 70° F, but can be produced with temperatures ranging from 50 to 80° F. Temperatures above 90° F, particularly with warm overnight temperatures, can result in poor pollen germination and flower drop.
Maturity
Beans are often produced for processing where specific quantities are required on specific dates. This can be partially handled by selecting varieties with different days to maturity. In addition, successive plantings can be made to ensure beans are mature when needed. Bush snap beans should mature in 48 to 60 days depending on variety. Runner or pole beans require 58 to 70 days to mature. Lima (bush) and pole beans require 65 to 80 days and 80 to 88 days, respectively.
Finally, growers can use growing degree days (GDD) to help determine when a crop will mature. This method uses the accumulated average daily temperature above a specific baseline to determine how long it will take a crop to mature from sowing to harvest.
For snap beans, the required GDD is 1,050 to 1,150 with a baseline temperature of 50° F. Using the above equation, the GDD for a single day with a minimum temperature of 60° F and a maximum of 80° F would be 20. If the next day had a minimum temperature of 64° F and a maximum of 86° F then that day's GDD would be 25 and the accumulated GDD for the two days would be 45.
½ûÂþÌìÌà maintains an automated environmental monitoring network (accessible at: http://www.georgiaweather.net/), which can be used to acquire daily minimum and maximum temperatures and will calculate the GDD.
For example, using the Vidalia weather station to calculate days to maturity for a planting in south Georgia on February 15, 2009, it would have taken until May 9 (83 days) to accumulate 1,047 GDD units. It should be noted, however, that GDD calculations during very cool weather tend to underestimate the days until maturity. With a more realistic planting date of March 10, the accumulated GDD would be 1,049 on May 14, 2009 — 65 days after planting. An April 1 planting date would have accumulated 1,039 GDD by May 26 — 55 days after sowing. Conversely, sowing on September 15, 2008 would have required until December 10 to accumulate 1,035 GDD — a total of 86 days from sowing to harvest.
It should be noted that the GDD method is an estimate and the accumulated GDD will change from one year to the next and will be slightly different for different varieties. It is not a precise estimate of the number of days to maturity and your experience with the crop under your growing conditions should allow you to make adjustments in interpreting these results and planning for future crops.
Harvest
Bush snap beans are harvested at the "mature green" stage, which is when the beans have attained the size for the specific variety and the pods are still tender. The concentrated pod set allows all of the beans to be harvested at once. Research has shown that for snap beans with a weight/length ratio of 0.4 gm/cm or less the pods will be very tender. A weight/length ratio of 0.41 to 0.5 gm/cm is tender and optimum for harvest. A weight/length ratio of 0.51 to 0.6 gm/cm is considered mature; ratios above this are considered over-mature. Beans should not be allowed to stay on the vine longer than this stage because the pods will become tough and fibrous. "Stringless" varieties have a reduced or delayed fiber development that makes the pods less prone to being tough and stringy at maturity.
Lima beans should be harvested when the pods are still green but the seed has filled out completely. Several pods should be shelled to assess seed fill before harvest to ensure good yields.
Runner or pole beans of either snap beans or lima beans are usually harvested over an extended period of time. There may be three to 10 harvests, which should occur every three to five days. Removing mature beans encourages immature pods to develop and increases flowering. Beans left on the plant will eventually mature to the dry bean state, reducing further flowering and "mature green" bean production.
Grading
Grading is a voluntary process that can help facilitate sales, particularly interstate or international sales. It assures buyers that they are getting a quality, uniform product. Snap beans for fresh market are graded into three classes based on USDA grade standards U.S. Fancy, U.S. No. 1 and U.S. No. 2. These grade standards are available at:
The grade standards are based on uniformity and overall quality for the specific variety, not on size. U.S. Fancy allows 10 percent of the lot by weight to be outside the grade requirement with not more than 3 percent due to broken pods and not more than 5 percent for serious damage, including 1 percent for soft decay. U.S. No. 1 beans are allowed to be 13 percent out of grade, with only 10 percent damage other than broken pods and no more than 5 percent from serious damage, including 1 percent from soft decay. Finally, U.S. No. 2 beans can have up to 15 percent out of grade, including 10 percent from serious damage but less than 1 percent from soft decay.
Lima bean grades can also be found at http://www.ams.usda.gov/AMSv1.0/Standards. Lima beans are also divided into three grades: U.S. No. 1, U.S. Combination and U.S. No. 2. As with snap beans, lima bean grades are based on varietal characteristics and uniformity; however, these grades apply to the pod. U.S. No. 1 should have varietal characteristics with no more than 10 percent off grade by weight. Not more than 5 percent of this grade can be from serious damage and not more than 1 percent can be from soft decay. U.S. Combination is a combination of U.S. No. 1 and U.S. No. 2 grades, with 75 percent by weight from U.S. No. 1. U.S. No. 2 also allows 10 percent by weight outside the grade with not more than 1 percent from soft decay.
Snap beans for processing are graded into two classes: U.S. No. 1 and U.S. No. 2. U.S. No. 1 beans have a maximum diameter of 24/64 inches (sieve No. 4) and a minimum size of 12/64 inches (sieve No. 1) (Table 2). U.S. No. 2 beans have a minimum diameter of 12/64 inches (sieve No. 1) and no maximum diameter.
Lima beans for processing are also graded into two classes: U.S. No. 1 and U.S. No. 2. U.S. No. 1 includes shelled beans that are green in color with similar varietal characteristics, free from decay and injury. U.S. No. 2 beans are free from decay and injury.
With all grading, growers should read and understand the grade standards. Growers may wish to consult their buyers and USDA personnel for clarification in order to handle grading properly.
Good yields should average 250 to 300 bushels (30 pounds) per acre. Under ideal conditions, yields as high as 400 to 450 bushels per acre are possible. Generally, yields will be lower with machine harvesting compared to hand harvesting.
| Table 1. Recommended varieties for Georgia. | ||||
| Snap beans | Lima beans | |||
| Bush | Pole | Bush (small seed) |
Bush (large seed) |
Pole |
| Ambra | Dade | Bridgeton | Fordhook 242 | Carolina Sieva |
| Bronco | McCaslan | Dixie Butter Pea | Dixie Speckled Butter Pea | Florida Speckled |
| Bush Blue Lake 274 | State (half runner) | Early Thorogreen | King of the Garden | |
| Caprice | Stringless Blue Lake | Henderson Bush | ||
| Carlo1 | Volunteer (half runner)2 | Nemagreen | ||
| Charon | White Seed Kentucky Wonder 191 | |||
| Festina | ||||
| Grenable | ||||
| Hickok | ||||
| Magnum | ||||
| Nash | ||||
| Renegade | ||||
| Roma II (flat pod) | ||||
| Storm | ||||
| Strike | ||||
| Valentino | ||||
| 1 Spring production only. 2 Not for Coastal Plain areas. |
||||
| Table 2. Sieve sizes for classifying snap beans for processing. | |
| Sieve size | Diameter |
| No. 1 | 12/64 to, but not including, 14.5/64 inch |
| No. 2 | 14.5/64 to, but not including, 18.5/64 inch |
| No. 3 | 18.5/64 to, but not including, 21/64 inch |
| No. 4 | 21/64 to, but not including, 24/64 inch |
| No. 5 | 24/64 to, but not including, 27/64 inch |
| No. 6 and larger | 27/64 inch and larger |
Soils and Fertility
George Boyhan, Extension Horticulturist - Vegetables
Bush beans (Phaseolus vulgaris) and lima beans (P. lunatus) are easy to grow, moderate feeders that require relatively low levels of nitrogen. A well-drained fertile soil is best for bean production, but good results are possible with soils that are not ideal. Drainage problems, however, should be corrected prior to planting to prevent soil-borne diseases and root respiration problems. High amounts of organic matter in conjunction with poor drainage can be particularly detrimental to seedlings.
Soils in Georgia are split into two broad categories: the Coastal Plain soils of south Georgia and the Piedmont, Mountain and Limestone Valley soils of north Georgia. The Coastal Plain soils are lighter sandy loam soils that generally drain quickly and warm early in spring. They are characterized as unstructured mineral soils with very little organic matter, which means they do not hold together by forming clods and are relatively infertile as cultivated soils. With proper management and fertilization, however, they can be very productive, and because they warm early and drain well they are ideal for vegetable production.
The Piedmont, Mountain and Limestone Valley soils tend to be heavier with a greater amount of clay content. In fact, the famous "red clay" of Georgia, which occurs because of iron oxides in the soil, belongs in this group. These soils are also generally infertile and have little organic matter, but because of the high clay content they are capable of holding more nutrients for a longer period of time; consequently, nitrogen recommendations are lower. A takes into account the differences between these soil types when recommendations are issued.
A should be done before planting to ensure the pH and soil fertility are optimum for maximum yield — 6.0 to 6.5 for beans. The should be taken several months prior to planting, especially if you suspect the pH is low. Correcting low pH can take several months after the application of lime.
Beans require 70 to 100 pounds of nitrogen per acre on the Coastal Plain soils of south Georgia and 60 to 80 pounds on the Piedmont, Mountain and Limestone Valley soils of north Georgia (Table 1). For best results, broadcast or band apply one-half of the nitrogen prior to sowing. This can be topdressed at planting or incorporated prior to sowing. The remaining one-half of the nitrogen can be applied in one or two additional applications three to six weeks after sowing.
Potassium should also be split-applied, with some applied at planting and the remainder applied during the crop cycle. Phosphorus application, on the other hand, should all be applied preplant since this nutrient is relatively immobile in the soil. A soluble form of phosphorus applied in solution such as 11-34-0 may aid early growth, particularly in early spring when temperatures are cool. This type of fertilizer application is often referred to as "pop-up" fertilizer.
Pole beans are running or vining types that produce 2- to 3-foot-long vines as opposed to bush types that stand erect and are usually no taller than 1 to 2 feet. The larger pole bean plant requires more nitrogen fertility, although the recommendations for pH, phosphorus and potassium will be the same as for bush type beans. The amount of nitrogen required is 100 to 140 pounds per acre on the Coastal Plain soils and 90 to 120 pounds per acre on the Piedmont, Mountain and Limestone Valley soils. Fertilizer application timing is the same as for bush beans.
In addition to nitrogen, phosphorus and potassium requirements, Georgia soils may require magnesium. If magnesium levels test low and pH is going to be adjusted, use dolomitic limestone since this also supplies magnesium. If no adjustment to the pH is required and magnesium levels are low, apply 25 pounds per acre of magnesium prior to sowing.
Georgia soils can be deficient in both sulfur and boron. Apply 10 pounds per acre of sulfur and 1 pound per acre of boron preplant. If zinc levels test low, apply 5 pounds per acre preplant.
Beans mature fairly quickly and are easy to grow so leaf tissue nutrient monitoring is generally not required. However, if a nutrient deficiency is suspected, tissue samples can be taken to determine if there is a problem. Table 2 lists the sufficiency ranges for bean. Samples of affected and unaffected leaves should be collected separately to send to the Soil, Plant and Water Analysis Laboratory in Athens for analysis. These samples should consist of the most recently mature leaves from representative plants.
Soil temperature is an important consideration when sowing beans. The minimum soil temperature should be 60° F, with optimum germination occurring at 75 to 80° F. If soil temperatures are too cool, bean seedlings are more prone to disease development, resulting in a poor stand.
In a comprehensive production program, crop rotation can help maintain and improve soil fertility. Beans can be an important part of such a system because of their ability to fix nitrogen from the atmosphere and make it available for both the current bean crop and subsequently planted crops.
| Table 1. Recommended potassium and phosphorous applications based on ratings of each nutrient.* | |||||
| Phosphorous Rating | Potassium | ||||
| Low | Medium | High | Very High | Overload | |
| (Pounds N-P2O5-K2O per acre) | |||||
| Low | -90-90 | -120-90 | -120-60 | -120-30 | -120-0 |
| Medium | -70-90 | -90-90 | -90-60 | -90-30 | -90-0 |
| High | -50-90 | -60-90 | -60-60 | -60-30 | -60-0 |
| Very High | -0-90 | -30-90 | -30-60 | -30-30 | -30-0 |
| *Nitrogen recommendations: Snap and Lima Beans - Coastal Plain Soils: 70-100 lbs/acre N; Piedmont, Mountain and Limestone Valley Soils: 60-80 lbs/acre N. Pole Beans - Coastal Plain Soils: 100-140 lbs/acre N; Piedmont, Mountain and Limestone Valley Soils: 90-120 lbs/acre N |
|||||
| Table 2. Plant tissue analysis critical values for snap beans.* | ||||||||||||
| Nutrient | Percent | ppm | ||||||||||
| N | P | K | Ca | Mg | S | Fe | Mn | Zn | B | Cu | Mo | |
| Deficient (<) | <3.0 | 0.25 | 2.0 | 0.8 | 0.20 | 0.20 | 25 | 20 | 20 | 15 | 5 | - |
| Adequate | 3.0- 4.0 |
0.25- 0.45 |
2.0- 3.0 |
0.8- 1.5 |
0.20- 0.45 |
0.21- 0.40 |
25- 200 |
20- 100 |
20- 40 |
15- 40 |
5- 10 |
0.4- 1.0 |
| High (>) | >4.1 | 0.46 | 3.1 | 1.6 | 0.46 | 0.41 | 200 | 100 | 40 | 40 | 10 | 1.0 |
| Toxic (>) | 1000 | 150 | ||||||||||
| *Adapted from Knott?s Handbook for Vegetable Growers, 5th Edition. | ||||||||||||
Irrigation
Kerry Harrison, Extension Engineer
Introduction
Because water management is so important, most commercially grown snap beans in Georgia are irrigated. Research and Extension trials in Georgia have indicated that properly irrigated snap beans will yield 25 to 50 percent more than dry land snap beans.
Irrigation System Options
Almost all snap beans in Georgia are sprinkler-irrigated. The two most commonly used systems are center pivots and traveling guns.
Center pivot systems are generally one of the lowest cost systems per acre to install and require very little labor to operate. When properly maintained, they apply water very uniformly. Because center pivots usually require a lower pressure than a traveling gun, they are generally very energy efficient. However, they are not well adapted to small, irregularly shaped fields, and unless the system is towable, it is restricted to use in only one field. If a farmer has a limited amount of irrigated land, this characteristic can be detrimental to desirable crop rotations.
Traveling guns are mobile systems that can be moved from field to field or farm to farm. They can be used on almost any sized or shaped field. However, they do require high water pressure to operate and consequently require more fuel per acre-inch of water than pivot options. Traveling guns also require a considerable amount of labor to operate. These systems tend to increase soil compaction and are harsh on young plants.
There has been interest in installing drip irrigation systems similar to those used in other vegetable production (bell pepper, tomato, etc.). The drip irrigation tubes are commonly referred to as "tape." These systems work very well when properly managed; however, they have very little history in Georgia related to snap bean production and should be used with caution. If you are familiar with managing this type of drip irrigation, you are well on the way to a better understanding of the situation. If you have never used drip irrigation, you need to consider very carefully what changes in your irrigation management may be necessary to be successful.
Irrigation Scheduling
Snap bean water use varies considerably throughout the growing period and based on weather conditions. The peak water demand can be as high as 1.5 to 2 inches per week. Peak use generally occurs during the latter stages of enlargement, especially during periods of warm weather; however, there are other stages when supplemental water may be needed.
Although the fall months tend to be some of the driest months in Georgia, irrigation applications are typically infrequent. This is because plants are relatively small particularly during early growth. During this period, irrigation should be applied whenever the top 6 inches of the soil becomes dry. Irrigation amounts should be limited to about 0.5 inch per application during this stage.
When the plants begin to fill fruit and the weather turns dry, the water demand and need to irrigate will gradually increase. Rooting depths at this stage are typically 12 inches or less. Because of the shallow rooting depth, irrigation applications should not exceed 1 inch. Typical applications should range between 0.6 and 1 inch for loamy and sandy soils, respectively. During dry weather, irrigate two or three times per week, especially when temperatures are warm. Of course, when temperatures are cool, irrigations may be less frequent.
Unlike most other crops, snap beans do not generally wilt when they experience moisture stress. Since moisture stress is difficult to detect by visual inspection, it is very helpful to monitor soil moisture. This can be done by installing tensiometers, electric resistance blocks, C-probes or other devices in the soil. Install soil moisture sensors at two depths — one near the middle of the root zone and one near the bottom. Common practice is to install one at 4 to 6 inches and one at 10 to 12 inches. The ideal range for soil moisture is between 5 and 30 centibars for most Coastal Plain soils. Readings less than five indicate saturated conditions and readings above 30 indicate the soil is becoming dry. If you use a center pivot or traveling gun, you should start irrigating early enough so that the last part of the field to get watered does not get too dry before the system reaches it.
In general, if the system requires three days to water the entire field, then you should install at least three soil moisture stations evenly spaced around the field. Each station will consist of two sensors — one shallow and one deep. You should monitor the readings on the soil moisture sensors at least three times per week when the weather is dry.
The equipment used for applying liquid insecticides, fungicides, herbicides and foliar fertilizers are classified as sprayers. Two types of sprayers — hydraulic and air-curtain boom — are recommended for spraying snap beans. The key to maximum coverage with insecticide and fungicides is replacing the air within the plant canopy with pesticides.
Sprayers
Paul E. Sumner, Extension Engineer
 Figure 1. Air-assisted sprayer for insect and fungicide application.
Figure 1. Air-assisted sprayer for insect and fungicide application.Air-curtain booms (Figure 1) are designed with an external blower fan system. The blower creates a high velocity of air that will "entrain" or direct the spray solution toward the target. Some sprayers provide a shield in front of or behind the conventional spray pattern to protect the spray from being blown off-target.
The concept of this approach is to increase the effectiveness of pest-control substances, provide better coverage to the underside of leaves, promote deeper penetration into the crop canopy and make it easier for small droplets to deposit on the target, cover more acres per load and reduce drift.
Studies conducted by the USDA Agricultural Research Service have shown that air-assisted sprayers tend to show improved insect control in the mid- to lower canopies. The air stream tended to open the canopy and help spray particles penetrate to a deeper level. Mid- to lower-canopy penetration and coverage is important when working with insecticides and fungicides.
 Figure 2. Hydraulic boom sprayer. The sprayer shown here is applying pesticides to tomatoes, but the spray is the same for snap beans.
Figure 2. Hydraulic boom sprayer. The sprayer shown here is applying pesticides to tomatoes, but the spray is the same for snap beans.Hydraulic boom sprayers (Figure 2) get their name from the arrangement of the conduit that carries the spray liquid to the nozzles. Booms or long arms on the sprayer extend across a given width to cover a particular swath as the sprayer passes over the field. Each component is important for efficient and effective application.
Most materials applied by a sprayer are a mixture or suspension. Uniform application demands a uniform tank mix. Most boom sprayers have a tank agitator to maintain uniform mixture. The agitation (mixing) may be produced by jet agitators, volume boosters (sometimes referred to as hydraulic agitators) and mechanical agitators. These can be purchased separately and put on sprayers. Make sure an agitator is on every sprayer. Some growers make the mistake of not operating the agitator when moving from field to field or when stopping for a few minutes. Always agitate continuously when using pesticides that tend to settle out.
Nozzles
Nozzle tips are the most neglected and abused part of the sprayer. Since clogging can occur when spraying, clean and test nozzle tips and strainers before each application. When applying chemicals, maintain proper ground speed, boom height and operating pressure to ensure proper delivery of the recommended amount of pesticide to the plant canopy.
Herbicides
The type of nozzle used for applying herbicides is one that develops a large droplet and has no drift. The nozzles used for broadcast applications include the extended range flat fan, drift reduction flat fan, turbo flat fan, flooding fan, turbo flooding fan, turbo drop flat fan and wide angle cone. Operating pressures should be 20 to 30 psi for all nozzles except drift reduction and turbo drop flat fans, flooding and wide angle cones. A spray pressure of more than 40 psi will create significant spray drift with flat fan nozzles. Drift reduction and turbo drop nozzles should be operated at 40 psi. Flooding fan and wide angle cone nozzles should be operated at 15 to 18 psi. These nozzles will achieve uniform application of the chemical if they are uniformly spaced along the boom. Flat fan nozzles should be overlap 50 to 60 percent.
Insecticides and Fungicides
When applying insecticides and fungicides, use solid or hollow cone type nozzles. The two patterns that are developed by solid or hollow cone nozzles can be produced by different tip configurations. One type tip, disc-n-core, consists of two parts: a core (swirl plate) where the fluid enters and is forced through tangential openings, and a disc-type hardened stainless steel orifice (opening). Another type of tip that produces the same patterns is made of one-piece construction (nozzle body). Liquid is passed through a precision distributor with diagonal slots that produce swirl in a converging chamber. The resulting pattern of both tip configurations is either solid or hollow cone. Even flat and hollow cone nozzles can be used for banding insecticide or fungicides over the row.
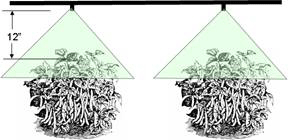 Figure 3. Use one or two nozzles over the row for small plants.
Figure 3. Use one or two nozzles over the row for small plants. Figure 4. As snap beans grow, use drop nozzles for additional penetration.
Figure 4. As snap beans grow, use drop nozzles for additional penetration.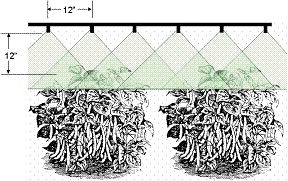 Figure 5. Nozzle configuration for broadcast spraying.
Figure 5. Nozzle configuration for broadcast spraying.Nozzle Arrangements
When applying insecticides and fungicides, it is advantageous to completely cover both sides of all leaves with spray. Use one or two nozzles over the top of the row (up to 8 inches wide) when spraying snap beans. As the plants start to grow and bush, the nozzle arrangement should be adapted for the various growth stages (Figures 3 and 4). Opposing nozzles should be rotated clockwise slightly so that spray cones do not collide. This will guarantee that the spray is applied from all directions into the canopy. As the plants increase in height, add additional nozzles for every 8 to 10 inches of growth. Broadcast spraying (Figure 5) should be done in cases when foliage between plants does not allow spray drops to be used. Penetration into the canopy will not be as effective as using drops (Figure 4). In all spray configurations, the nozzle tips should be 8 to 12 inches from the foliage. Properly selected nozzles should be able to apply 25 to 125 gallons per acre when operating at a pressure of 60 to 200 or higher psi. Usually, more than one size of nozzle will be needed to carry out a season-long spray program.
Calibration
Calibration should be conducted every eight to 10 hours of operation to ensure proper pesticide application. A good calibration procedure to follow can be found in "Calibration Method for Hydraulic Boom and Band Sprayers and Other Liquid Applicators," University of Georgia Extension Circular 683. This circular is available through local county Extension offices and on the Web at www.caes.uga.edu/publications.
Diseases
David B. Langston, Jr., Extension Plant Pathologist
Many diseases cause considerable damage to snap beans each year. Most of these diseases are common among leguminous hosts in general, therefore limiting the beneficial aspects of rotation in many cases. Each disease requires a certain growth stage or environmental condition to be successful at causing disease. It is up to the grower, agent or consultant to be aware of the times and conditions that are critical to disease development and take the necessary steps to prevent losses.
Seedling and Strictly Soilborne Diseases
Many pathogens attack snap beans early in their development and are generally referred to as seedling diseases. For the most part, these are soilborne diseases that are already in the field before seed are planted. Soilborne diseases indicate that the pattern of disease may be clumped and non-random and may follow a particular pattern in the field based on inoculum density, drainage and soil type.
Pythium Damping-off
Damping-off caused by Pythium spp. is one if not the most common seedling diseases. This disease can build up in soils that are heavily cultivated to legumes and is worse in soils that tend to be wet or poorly drained. Many Pythium spp. seem to thrive at cooler temperatures while others do well in warmer climates. Pythium spp. can cause seed rot, as well as pre- and post-emergence rots. Lesions generally appear water-soaked and may contain white, hair-like mycelium. Seedlings that emerge may wilt and die while standing, with only a small portion of the lesion visible at the soil line.
Rhizoctonia Damping-off and Tip Blight
Another damping-off disease is caused by the fungus Rhizoctonia solani, which can build up in soils heavily grown in legumes as well as on other crops. This pathogen attacks during moderate temperatures and soil moisture plays little role in disease onset and severity. The lesions are reddish, dry and sunken on hypocotyls, and plants may often lodge at the point of infection. A tip blight on bean pods can be observed as sunken, dry lesions later in the season.
Southern Blight
Sclerotium rolfsii (the Southern blight fungus) has also been involved in some early season stand loss. Infection by Southern blight is favored by high temperature and humidity. Lesions appear dark brown, sunken and sometimes water-soaked. Stringy, white mycelium is very noticeable on infected tissue. This disease is of minor importance compared to the two aforementioned diseases and generally causes disease later in the season on larger plants.
Disease Management
To reduce losses to seedling diseases, the University of Georgia recommends an integrated approach that utilizes cultural, varietal, sanitation and chemical tools.
- Cultural Management — Rotation with non-legume crops reduces potential inoculum of all seedling diseases. Select sites that are well-drained and that can be irrigated without excess soil water retention.
- Varietal Management — Choose varieties that have good seedling vigor and that are recommended for planting in Georgia.
- Sanitation Management — Deep-turning soil buries inoculum and reduces seedling disease pressure from Southern blight and Rhizoctonia damping-off. Pythium is not affected by deep turning. Do not throw infested soil against the bean stems during cultivation since this increases the likelihood of infection by exposing more plant tissue to infested soil.
- Chemical Management — Chemicals containing mefenoxam (Ridomil), PCNB (Terrclor) and azoxystrobin (Quadris/Amistar) used at planting will suppress losses to seedling diseases. Mefenoxam fungicides suppress diseases caused by Pythium spp. while PCNB and azoxystrobin suppress Rhizoctonia damping-off and Southern blight. Combination products must be used to encompass all seedling diseases. Azoxystrobin used after pod set will aid in suppressing losses to the crown rot and tip blight phases of Southern blight and Rhizoctonia tip blight.
Foliar Diseases
Most snap bean diseases are airborne and attack the plant after the second trifoliate stage. Airborne diseases like rusts travel many miles before they cause field epidemics. There are also nearly as many soilborne diseases, which splash up on above-ground plant parts and cause infections.
Bean Rust
Rust, caused by the fungus Uromyces appendiculatus, attacks all above-ground parts of the bean plant, but is most commonly seen on the underside of the leaves. The symptoms on the upper leaf surface appear as small, necrotic lesions surrounded by a yellow halo. On the underside of the leaf, corresponding with the upper leaf surface lesions, fuzzy, rust-colored pustules can be observed. Warm, humid temperatures and long day lengths favor disease development. Spores are wind-dispersed over long distances.
Disease Management
- Cultural Management — Rotation with non-legume crops and residue destruction helps reduce spread from initial inoculum.
- Varietal Management — This is probably the most important rust control measure, as many commercial varieties have good resistance to rust and acceptable horticultural characteristics.
- Chemical Management — Many protectant and systemic chemicals are available and effective on rust; see the current edition of the Georgia Pest Management Handbook.
Anthracnose
Anthracnose, caused by the fungus Colletotrichum lindemuthianum, is favored by cool, wet weather. It may cease to be active during hot, dry weather. It is more common in north Georgia than in other parts of the state, and occurs more commonly in home gardens where locally grown seed are used. Symptoms include brick red to purple lesions running along leaf veins. Infected pods may have sunken, tan to rust-colored cankers. This fungus overwinters on diseased bean plants left in the field after harvest and can live in seed as long as they remain viable. Spores will survive in old bean debris under field conditions for more than two years. Once the disease is brought into a field on the seed, it can be spread by splashing rain and insects, or by people and equipment when the beans are wet.
Disease Management
- Disease-Free Seed — Seed grown in dry regions of the world have a lower chance of being infected and should always be used in preference to locally produced seed.
- Crop Rotation — Do not grow beans on the same soil more often than once every three years. This fungus survives in old plant material in the field, but is not usually spread to new, uninfected sites from the old, infected debris.
- Varietal Management — Some cultivars have resistance to this pathogen, but disease may still occur on resistant lines due to variations in pathogenicity of this fungus.
- Chemical Management — Many protectant and systemic chemicals are available and effective on anthracnose (especially strobilurins and thiophanate methyl) and are listed in the Georgia Pest Management Handbook.
Pythium Cottony Leak (Aerial Pythium)
Pythium Cottony Leak (usually caused by Pythium aphanidermatum) has become one of the most widespread and destructive diseases of snap bean in Georgia. This disease produces profuse, white, aerial mycelia that look like cotton. Generally, this disease is favored by hot, wet weather.
Disease Management
- Crop Rotation — Avoid close rotations behind other legume crops like beans, peanuts, peas and soybeans.
- Sanitation Management — Avoid contaminating harvested beans with diseased beans as this disease can spread rapidly once boxed and shipped.
- Water and Nutrient Management — Avoid over-irrigation and fertilization as excessive moisture and lush plant growth create a favorable environment for this disease. Promote good drainage in fields to avoid standing water or excessive surface water retention.
- Spray Management — Ridomil Gold Copper has a 24C label for suppressing this disease in Georgia.
White Mold
This disease is caused by the fungus Sclerotinia sclerotiorum. It is often confused with Pythium cottony leak and both organisms produce very similar (if not identical) white mycelia. However, Pythium cottony leak causes disease during the summer when it is hot, and white mold is a problem primarily during late fall or early spring. Upon microscopic inspection, mycelia from white mold are septate while those from Pythium cottony leak are not. White mold will also produce irregular-shaped, black, hard sclerotia, which are characteristic of the causal fungus. Lesions from white mold are very tan and shred easily when disease tissue is dried.
Disease Management
- Crop Rotation — Avoid close rotations in fields with a history of severe losses to this pathogen. Since this fungus is a pathogen of many crops, avoiding only legume crops does not aid greatly in reducing inoculum.
- Sanitation Management — Avoid contaminating harvested beans with diseased beans as this disease can spread rapidly once boxed and shipped. Deep turning will bury and eliminate much of the inoculum.
- Water and Nutrient Management — Avoid over-irrigation and fertilization as excessive moisture and lush plant growth create a favorable environment for this disease.
- Spray Management — There are several fungicides labeled for suppressing this disease: Endura (boscalid), Rovral (iprodione) and Tospin (thiophanate methyl). Preventively spraying fields with a history of disease is recommended, and scouting fields and spraying at disease onset often helps.
Viruses
A number of viruses, including common bean mosaic, Southern bean mosaic and yellow bean mosaic, may affect snap beans. Common bean mosaic and Southern bean mosaic may be seed transmitted. All three virus diseases may be spread by insects. Yellow bean mosaic is usually spread from clover fields to beans by aphids, and is found only on pole beans in north Georgia. It is usually widespread only where beans are planted after clover or adjacent to clover fields. Mosaic is considered a minor bean disease in Georgia, except in North Georgia near clover fields.
Symptoms
Symptoms of the different virus diseases are very similar and difficult to separate. All the mosaic virus diseases cause some stunting and reduced yields. The affected bean leaves have irregular, light green or yellow areas merging with darker green patches, which produce the familiar mottling or mosaic effect. The mottling of contrasting yellow and green areas serves as a means of distinguishing yellow bean mosaic from common bean mosaic.
Plants infected by yellow bean mosaic are more dwarfed and bunchy. Bean pods on infected plants are quite often undersized and sometimes curled. They may contain fewer beans than those produced on normal plants. Leaves may be long and narrow and show some puckering or downward cupping at the margin. The overall symptoms may differ slightly with the variety and age of the plants and, to some extent, with growing conditions.
Disease Management
- Rotation — Fields planted to beans or red clover should not be planted back to beans the following year.
- Sanitation Management — Clean up all weed host plants adjacent to the bean field that could serve as a reservoir for the virus.
- Certified Seed — Since common bean mosaic and Southern bean mosaic may be seed transmitted, use certified seed.
- Resistant Varieties — Use resistant varieties when available. Disease resistance will be listed on the variety description in a seed catalog.
Root-knot Nematodes
Several different nematodes, including root-knot (Meloidogyne spp.), lesion (Pratylenchus spp.), sting (Belonolaimus spp.), stubby-root (Trichodorus spp.) and cyst (Heterodera spp.) may affect snap beans. All parasitic nematodes cause similar above-ground symptoms on beans.
Root-knot is probably the most common nematode and causes the most damage to snap beans in Georgia. Due to the buildup of nematodes during the summer months, fall beans are more apt to be severely affected than spring beans. Recommended bean varieties are susceptible to root-knot nematodes. If a high population of root-knot is present in the soil, beans may be stunted, produce low yields or be killed. Root-knot damage is usually more severe on light, sandy soils.
Symptoms
Usually, root-knot nematodes are not evenly distributed over a field; thus, symptoms may appear in round to irregular patches in a field. The most noticeable symptom may be irregular plant height and vigor.
Nematodes damage plant roots. Above-ground symptoms are those of a plant with a damaged root system: pale green to yellow foliage, stunting and abnormal wilting during the warmer part of the day. Below-ground symptoms furnish the positive proof of root-knot problems. Affected roots have swellings or galls, ranging from very small pinhead size to .05 inch or more in diameter on the larger roots. These galls are enlargements of the root tissue and cannot be detached without breaking the root.
If the roots are not knotted but the above-ground symptoms indicate a nematode problem, ask your county agent to take a soil sample in the root zone of the affected plants to send to the Plant Disease Clinic in Athens to be checked for other types of nematodes that may also damage beans.
Disease Management
- Rotation — Rotations have some value if a single species of nematodes is present. If several genera or species are in a field, it is difficult to find a rotational crop that will not favor the buildup of at least one kind of nematode. Do not plant fall beans behind a root-knot-susceptible crop, such as cucumbers, tobacco, cotton, squash, tomatoes, cantaloupes, peas or beans. Leaving the land fallow during the summer or planting rye in the field in the fall should reduce the root-knot nematode population.
- Fumigation — Applying a fumigant-type nematicide (e.g., Telone II) in the row before planting is the best way to control nematodes in beans. Fumigants require a two-week waiting period between application and planting. The non-fumigant Mocap may be used at seeding. Mocap should not be used under heavy root-knot pressure situations.
Insect Management
Alton "Stormy" Sparks, Extension Entomologist
Insects can present problems in snap bean production from planting to harvest, but generally present the greatest concerns from bloom to harvest, when the marketable bean is developing. The most common insect problems in Georgia snap bean production are defoliators (primarily caterpillars) and pod feeders (primarily corn earworm and stink bugs). In most areas of Georgia, these problems are not consistent enough to warrant preventative treatments (some other states recommend an automatic treatment at pin stage). Thus, close monitoring of pest populations and applying insecticides only as needed provides the most economical control. Some counties experience severe whitefly pest pressure every fall, and this situation may justify preventative treatments. Fields with a history of specific pest problems or conditions known to be suitable for specific pests may also justify preventive treatments.
Tolerances for direct pests (those that attack the marketable portion of the plant) are much lower than tolerances for indirect pests (insects that feed entirely on foliage or other non-marketable parts of the plant). Prior to bloom, weekly pest monitoring may suffice, with twice weekly monitoring thereafter. The threshold for defoliating insects is 20 to 25 percent defoliation prior to bloom or 10 percent during podding, combined with continued insect pressure capable of additional defoliation. Identification of the insects contributing to defoliation is important for insecticide selection. Pod-feeding insects cannot be tolerated, with insecticide treatments recommended for pod-feeding caterpillars at one per 3 feet of row or 3 percent or more pod damage.
Proper identification of insects is essential for determining damage potential and selecting insecticides when needed. Insect pests that are important for commercial snap bean production can be divided into three groups: soil pests that attack germinating seeds, roots and young seedlings; foliar pests whose primary damage is removal of leaf tissue; and pod pests that directly feed on pods and seeds.
While several insects can feed on snap bean seedlings, the primary pests of concern are cutworms and the lesser cornstalk borer. Important foliar pests include lepidopteran larvae (e.g., corn earworm, loopers, green cloverworm) and, to a lesser extent, beetles (e.g., Mexican bean beetle, bean leaf beetle). Spider mites have become a more consistent foliar pest of snap beans in recent years, but still are considered occasional pests. Whiteflies are a serious annual pest for beans produced in the fall in the south-central counties of Georgia and have extended their area of influence in recent years. Pod-feeding insects, which have the most potential for economic damage, include stink bugs and corn earworm.
Pod-feeding Insects
Insects that directly attack bean pods have the greatest potential for crop damage. In Georgia, this group consists primarily of the corn earworm and stink bugs. However, even these pests typically require only two to three insecticide applications and may not require any treatment in some fields. Broad spectrum insecticides are frequently used for these pests to ensure adequate control of both groups. Thus, applications targeting these pests likely provide control of many of our occasional pests, but can also contribute to development of secondary pests such as spider mites.
Corn Earworm
The corn earworm, Helicoverpa zea, is a caterpillar pest with an extensive host range that can attack beans at almost any growth stage. They are capable of feeding and reproducing on foliage, but are of greatest concern after pod set when they will feed on the developing pods and seeds. Eggs are laid individually on leaves and hatch in three to five days. The larvae can feed on pods shortly after hatching. Early instar larvae have stout hairs that give them a somewhat spiny appearance as compared to the smooth skin of most other caterpillars found on snap beans. Older larvae vary greatly in color from a light green to brown or nearly black and are lighter on the underside. These larvae are marked with alternating light and dark stripes running lengthwise on the body. Larvae will consume portions of multiple pods, which allows for increased damage (versus development in or on a single pod) but also involves movement within the plant, which aids in exposure to pesticides. Corn earworm populations generally are low in the spring and increase throughout the year. This pest seldom requires treatment prior to bloom, but should be closely monitored during pod development. The suggested threshold for pod-feeding caterpillars is one per three feet of row or 3 percent of pods damaged.
Stink Bugs
Several species of stink bugs and related insects will feed on bean pods. Stink bug adults are generally medium sized shield-shaped bugs with broad "shoulders" and a bluntly rounded abdomen. They also have a triangular-shaped shield on their backs. The most common species encountered are a uniform green color (Southern green stink bug, Nezara viridula), or tan to brown with a light-colored underside (several species of brown stink bugs, Euschistus sp.). A related group, the leaffooted bugs (Leptoglossus sp.), are brown, medium-sized bugs that get their common name from the flattened segment of the hind leg, which has a leaf-like appearance.
Stink bug nymphs are more oval-shaped than adults and vary greatly in color. Eggs are somewhat barrel-shaped and are deposited in tightly packed clusters. Nymphs and adults have needle-like mouthparts with which they puncture plant tissue and remove sap. The greatest damage occurs when they feed directly on seeds within the pod. Feeding on newly developing seeds results in small, shriveled seed, while feeding late in seed development may result in little more than a discolored spot on the seed. Thus, potential damage varies with crop stage. Stink bugs rarely require control prior to podding. Once seed begin forming, the suggested thresholds range from one stink bug per 1 foot of row to one per 3 feet of row.
European Corn Borer
European corn borer does occur in snap beans in Georgia, but is more of a regulatory pest than a direct economic pest. European corn borer populations are generally low in Georgia and are generally controlled by insecticides applied for corn earworm and stink bugs. It is of regulatory concern for those growers wishing to ship produce to specific states (currently Texas, California, Washington and Nevada). Participation in the program for shipping to these states requires records of preventative insecticide treatments and phyto-sanitation certificates. Some states also require pheromone trapping to monitor adult densities. Growers wishing to participate in this program should contact the Georgia Department of Agriculture, Plant Protection Division, prior to planting their crop for details on program costs and specific requirements.
Foliage-feeding Insects
A variety of arthropod pests will feed on snap bean foliage, but are generally considered occasional pests in Georgia since populations do not require insecticide treatments every year. However, several of these species can cause severe defoliation and loss of yield when the environment favors rapid population increases.
Caterpillars and Beetles
Several species of caterpillars and beetles can contribute to snap bean defoliation. Caterpillar species of concern include the soybean and cabbage loopers Pseudoplusia includens and Trichoplusia ni, respectively, beet armyworm, Spodoptera exigua, and green cloverworm, Hypena scabra. Beetles that may contribute to defoliation include the bean leaf beetle, Cerotoma trifurcata, and the Mexican bean beetle, Epilachna varivestis. All of these insects have chewing mouthparts and remove foliage from the plants. Control decisions are based on defoliation levels and continued presence of damaging insects. The threshold for defoliating insects is 20 percent defoliation prior to bloom or 10 percent during podding, combined with continued insect pressure capable of additional defoliation. Identification of the insects contributing to defoliation is important in insecticide selection. Some insecticides may provide excellent control of caterpillar pests but be a poor choice for a mixed population of caterpillars and beetles.
Spider Mites
Twospotted spider mites, Tetranychus urticae, are an occasional pest of snap beans in Georgia. Spider mites generally feed on the underside of leaves, but can cover the entire leaf surface when populations are high. The minute eight-legged mites appear as tiny reddish, greenish or yellow moving dots on the undersides of leaves. Because of their size, the first indication of spider mite infestations is usually damage to the leaves. Leaves infested with spider mites are initially lightly stippled with pale blotches. In heavy infestations the entire leaf appears light in color, dries up and may be covered with webbing. The greatest damage occurs during dry, hot weather that is favorable for rapid development of extremely high mite populations. Spider mites are also generally considered a secondary pest, with damaging populations frequently occurring after application of broad spectrum insecticides. To check for spider mites, observe plant foliage for characteristic damage. Look on the undersides of leaves for mites. Pay close attention to field borders and weedy areas. Mite populations frequently get started and reach their highest densities along field margins adjacent to roads where the plants are covered with dust. In general, treatments for mite control should be applied when mites become numerous and their damage appears excessive. Suggested thresholds range from 10 to 20 mites per leaflet.
Whiteflies
The sweetpotato whitefly, Bemisia tabaci, (a.k.a. silverleaf whitefly) is a pest in fall plantings of snap beans in some areas of south Georgia. In areas where this pest occurs, it can reach devastating densities in hot, dry years. Heavy populations can result in plant death. Lesser impacts include plant stunting, reduced yield and discoloration (bleaching) of pods. While the majority of snap bean acreage is not produced in areas with the greatest whitefly problems, this pest has expanded into these regions in recent years.
Adult whiteflies are tiny insects (about 1/8 inch) with powder-white wings and a yellow body. Adults occur primarily on the undersides of leaves, where they feed and lay eggs. The scale-like nymphs also occur on the undersides of leaves and all but the first instar are sessile (stationary). Adults and nymphs have piercing-sucking mouthparts, but the nymphs are considered more damaging. This insect also produces honeydew, which supports the growth of sooty mold when populations are high.
The silverleaf whitefly has an extremely large host range and presents the greatest challenge in areas with large acreage of vegetables and row crops, which provide year-round hosts for this pest. Spring vegetables have few problems, but whitefly populations can build up in summer crops and move into fall planted beans in overwhelming numbers. In these areas, preventive treatments are justified. Under less pest pressure, treatments are recommended when populations exceed three to five adults per leaf. Insecticides recommended for whiteflies vary greatly in the insect stages they control (eggs, nymphs or adults) and recommended timing of application (preventive versus curative).
Thrips
Several species of thrips may attack beans, with tobacco thrips being the predominant species on seedlings and various flower thrips predominating in blooms. Thrips are elongate, tiny (adults about 1/16 inch), dark to straw colored insects. Nymphs are white to yellowish in color. Plant injury is caused by both adults and nymphs, which puncture leaf and floral tissues and feed on exuding sap. Thrips are primarily of concern in beans when they invade plants early in development. Feeding on foliage first appears as silvery speckled areas on the leaves. Extensive damage to seedlings results in twisted, discolored and misshaped foliage. This type of damage is most likely to occur under cool weather conditions when plants are growing slowly. Beans will generally grow out of this damage once growing conditions improve and treatments are not required under normal growing conditions. If plants are under drought stress or are growing slowly (due to cool weather), control of thrips on seedlings may be justified. Thrips also appear in blossoms, but the potential for injury to the crop from these infestations is poorly understood. Thrips populations of five to 10 per bloom may justify insecticidal control.
Soil Pests
While a variety of soil-dwelling insects can attack snap beans, most are of minor concern in Georgia. The exception to this is the lesser cornstalk borer, which can significantly reduce plant stands in individual fields. However, pest populations are generally not consistent enough to justify preventive applications of pre-plant insecticides, which are the only efficacious controls for this pest.
Lesser Cornstalk Borer
The lesser cornstalk borer, Elasmopalpus lignosellus, is a consistent but minor pest of snap beans in Georgia. This small caterpillar attacks the main stem of seedling plants. The caterpillar bores into the main stem just below the soil line and will bore up the stem. The caterpillar forms a web tube that is usually attached to the plant just below the soil line. Boring within the main stem results in plant death of small plants and a greatly weakened stem in larger plants, leading to lodging. Lesser cornstalk borer is primarily a problem in late summer and fall plantings in sandy soils. Hot, dry weather also contributes to this pest's occurrence. While it appears as a problem in 10 to 15 percent of the acreage, the problem is generally not consistent and severe enough to justify preventive applications of a soil insecticide prior to planting. Once the problem appears in the crop, insecticide applications are generally ineffective because of the protective habitat of this species.
Grubs, Wireworms and Other Soil Pests
White grubs, wireworms, cutworms and other soil-inhabiting insects can cause stand reductions in areas within a field but seldom affect entire fields. Cutworms can be controlled with foliar insecticides, but most soil-inhabiting seedling pests require preventive treatments at, or prior to, planting. Most of these pests are a greater concern when planting into weedy or fallow fields; thus, field sanitation is important in managing these pests.
Seedcorn Maggot
Seedcorn maggot is a rare pest of snap beans but can have severe effects on stand establishment under certain conditions. This fly, which looks like a small housefly, is attracted to decaying organic matter. Eggs are placed in the soil and the larvae will feed on germinating seeds and emerging seedlings. Under severe pest pressure, this can result in loss of stand, requiring replanting. This occurs when beans are planted in a field with high organic matter (e.g., cover crop recently plowed under) and poor plant growing conditions (e.g., cool weather). Under good growing conditions (warm soil), beans generally emerge and establish too quickly for seedcorn maggot to impact stand. If planting following a cover crop, the cover crop should be plowed down three to four weeks before planting. If planting into cool soils with high organic matter, a preventive insecticide application after planting (to prevent oviposition) may be justified.
Other arthropods that may attack snap beans include aphids, cucumber beetle, tarnished plant bug, vegetable leafminer and potato leafhopper. Any of these insects can cause substantial damage to snap beans if conditions favor insect populations, but these conditions are rare in Georgia.
Weed Control
A. Stanley Culpepper, Extension Agronomist - Weed Science
Effective weed management is one of many critical components of successful snap bean production. Weeds compete with snap beans for light, nutrients, water and space, and can interfere with harvesting practices. Additionally, weeds can harbor deleterious insects and diseases. Severe weed infestations can eliminate snap bean production if the weeds are left uncontrolled.
Summer annual weeds, including yellow and purple nutsedge, morning glory, purslane species, nightshade, pigweeds and annual grasses, usually cause problems in snap beans.
One of the most effective tools for suppressing weeds in snap bean is a healthy, vigorous crop. Good crop management practices that result in rapid snap bean canopy development help minimize the effects of weeds.
Cultural Control Methods
Weeds can be controlled effectively through cultural practices that result in rapid snap bean canopy establishment, thus providing an undesirable environment for weed growth. Cultural practices may include the following: 1) planting weed-free seeds; 2) good seedbed preparation; 3) proper fertilization and watering; 4) following recommended row spacing; and 5) managing diseases and insects.
Site selection also can play a significant role in weed management. Rotation away from fields infested with troublesome weeds may minimize the presence of these weeds and allow for the use of alternative crops and control methods. Additionally, to prevent weed spread from field to field during harvest, equipment and personnel should be cleaned when moving from heavily infested areas. This precaution can be of significant consequence in preventing or minimizing the introduction of new weed species into "clean" areas.
Mechanical Control Methods
Mechanical control methods include field preparation by plowing or discing, cultivating, mowing, hoeing and hand pulling weeds. Mechanical control practices such as cultivation and primary tillage are often used in snap bean and are very beneficial for managing weeds. When using a systems approach that includes both herbicides and cultivation, it is important to remember that the cultivation process most often destroys the activity of residual herbicides.
Developing an Herbicide Program
Before selecting herbicides, growers should know what weeds are present or expected to appear, soil characteristics (such as texture and organic matter content), the capabilities and limitations of the various herbicides, and how best to apply each herbicide.
Weed Mapping
The first step in a weed management program is to identify the problem; this task is best accomplished by weed mapping. Surveys should be conducted each fall to provide a written record of the species present and their population levels. Weeds with the highest population levels in the fall will most likely be the most common weeds in the following spring-planted crop.
In-season Monitoring
Fields should be monitored periodically to identify the need for postemergence herbicides. Even after herbicides are applied, monitoring should continue to evaluate the success of the weed management program and to determine the need for additional control measures.
Proper weed identification is necessary since weed species respond differently to various herbicides. For assistance identifying weeds, contact your local county Extension office.
Stale Seedbed
The use of a stale seedbed approach prior to planting snap bean can be extremely useful in some situations. A stale seedbed approach is one where the land is prepared two weeks or more before planting. Preparing the land allows for the greatest weed flush to occur prior to planting the crop, thereby allowing the use of non-selective herbicide (glyphosate or paraquat, usually) to control emerged weeds at or prior to planting.
Herbicides
Properly selected herbicides are effective tools for weed control. Herbicides may be classified several ways, depending on how they are applied and their mode of action in or on the plant. Generally, herbicides are either soil-applied or foliage-applied. They may be selective or non-selective, and they may be either contact or translocated through the plant. For example, paraquat (Gramoxone) is a foliage-applied, contact, non-selective herbicide, while metolachlor (Dual) usually is described as a soil-applied, translocated, selective herbicide.
Foliage-applied herbicides may be applied to plant leaves, stems and shoots. Herbicides that kill only those parts of plants that the spray touches are contact herbicides. Those herbicides that are taken into and moved throughout the plant are called translocated herbicides. Paraquat (Gramoxone) is a contact herbicide, while glyphosate (Roundup) and sethoxydim (Poast) are translocated herbicides.
For foliage-applied herbicides to be effective, they must enter the plant. Good coverage is critical and these products often require the addition of some type of adjuvant. Soil-applied herbicides are either applied to the surface of the soil or incorporated into the soil. Lack of moisture or rainfall following application of soil-applied herbicides often results in poor control.
Many herbicides applied in Georgia offer residual weed control, which is beneficial in the crop where the herbicide was applied. However, before applying any herbicide in a crop, one must review the herbicide label and obtain the needed information on rotation restrictions. Many herbicides applied in peanut, cotton, tobacco and other vegetable crops can cause significant crop injury to snap bean planted the following year.
For herbicide recommendations, contact your local Extension office or view the most recent Georgia Pest Management Handbook.
Controlling Various Troublesome Weeds in Snap Bean
NOTE: This discussion on herbicides for use in snap beans is based on the current herbicide labeling for 2010. Refer to the most recent herbicide labels or your local county Extension office to determine that these recommendations are currently labeled and recommended for use in Georgia snap bean. Additionally, refer to the most recent label or the Georgia Pest Management Handbook for application timings, rates and restrictions (especially rotational restrictions) for the herbicides discussed below.
Pigweed Species
Fortunately, numerous tools exist to control pigweeds in snap bean. All growers should consider using trifluralin (Treflan, others), pendimethalin (Prowl, others), Dual Magnum or Reflex in fields infested with pigweed. Incorporated applications of trifluralin or pendimethalin are strongly encouraged, while Dual Magnum is often most effective when applied immediately after planting as a preemergence application and activated by irrigation or rainfall.
In severe pigweed infestations, an incorporated application of trifluralin or pendimethalin followed by a preemergence application of Dual Magnum has proven extremely effective (be careful with the herbicide rates with this program). Preemergence applications of Reflex are also extremely effective in managing pigweed; however, Reflex will not provide control of most grasses and many other broadleaf weeds so the addition of Dual magnum, Treflan or Prowl is needed in a Reflex-based weed management program.
Controlling pigweed with postemergence herbicides is much more challenging. Both Reflex and Sandea can be used to control some pigweed species. In general, Reflex is more effective and will control most pigweeds up to 3 inches in height. Reflex should be applied only once in a snap bean crop.
Sandea and Reflex pose significant carryover risk to some vegetables planted after snap bean; thus, rotational intervals for these herbicides should be studied prior to application.
Wild Radish and Nutsedge
Sandea is the most effective tool in snap bean to control these pests. Preemergence or postemergence applications of Sandea will often eliminate wild radish. For nutsedge, Sandea postemergence provides excellent control, while Sandea preemergence provides only fair control for about three weeks. In cool conditions, Sandea can cause snap bean chlorosis and a minor delay in maturity.
Common or Pink Purslane
Controlling purslane depends heavily on the use of at-plant residual herbicides. Trifluralin (Treflan, others) or pendimethalin (Prowl, others) preplant incorporated are the most effective options. Dual Magnum applied preemergence will also provide good control.
Morningglory
Reflex applied postemergence is the most effective tool to control morningglory species and should be applied when morningglory is less than 4 inches tall.
The author would like to thank Alan C. York (N.C. State University) and Bill Stall (University of Florida) for their contributions to this publication.
Food Safety and Sanitation
Bill Hurst, Food Science and Technology
Introduction
As produce consumption has increased in the past two decades, so have the number of foodborne illness outbreaks attributed to fresh produce. In 1998, the U.S. Food and Drug Administration published its guide for the fresh produce industry, Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables, also known as the Good Agricultural Practices (GAPs) document. This document was the first of its kind to focus on safe production and handling of raw agricultural commodities. GAPs identifies the many ways that pathogens harmful to humans can contaminate fresh produce, either through direct contact (infected workers or animals) or indirectly through contaminated soil, water, field or packing shed equipment. This document acknowledges that elimination of these pathogens is not possible except through "cooking the produce, or perhaps by irradiation."
However, GAPs does suggest some preventive strategies that a grower might use to reduce these risks before fresh snap beans leave the farm. This chapter focuses on ways to reduce contamination.
Pre-harvest
Land Use History
The safety of snap bean production is not only influenced by current agricultural practices, but by former land use practices. Human pathogens and pesticide residues can persist in soils for long periods of time. There may be an increased risk of soil contamination if production acreage was previously used as an animal feedlot or for grazing livestock. Production land should also be investigated to ensure that it was not formerly used for hazardous waste disposal or for industrial purposes, which may have left behind toxic residues.
Before planting, soils should be tested for high levels of heavy metals and for E. coli bacteria, an indicator of fecal contamination, as well as for pesticide residues. Produce buyers may insist on letters of guarantee from growers/packers that the land is suitable and safe for snap bean production.
Wild and Domestic Animal Control
 Figure 1. Pets should not be allowed in produce fields, especially near harvest time.
Figure 1. Pets should not be allowed in produce fields, especially near harvest time.Since most pathogens harmful to humans are carried by wild (birds, feral pigs, deer, raccoons, amphibians, etc.) and domesticated (livestock, dogs, cats, etc.) animals, selection of snap bean production fields must be done carefully. Be aware of land use near the field. Avoid establishing a snap bean field near or adjacent to animal operations, waste-handling facilities, livestock pastures or dusty roads where drift and water run-off could increase the risk for produce contamination. While it is impractical (and impossible) to keep wild animals out of open production fields, eliminate the contact of children and domestic animals, such as dogs, other pets, etc., especially near harvest time. Remove any animal material (waste, carcasses, etc.) from fields immediately, if possible and practicable. Workers who come in contact with live animals, animal carcasses or animal waste should wash any affected area (hands, boots, etc.) carefully before returning to work to prevent spreading the contamination.
Irrigation Water
Irrigation water is an important vehicle by which human pathogens and pesticides may be brought into contact with fresh snap beans. Deep well water is less likely to be contaminated by human pathogens than surface water if the well head and casing are properly maintained and livestock are excluded from the active recharge area. Natural surface water (e.g., lake, canals, ponds) provides enough organic matter and is routinely visited by wildlife so that the risk increases for the presence of human pathogens. Irrigation systems must be kept free of contaminants like scum buildup, human or animal waste products, etc. If a pump becomes contaminated, any water passing through it will spread the contamination onto the snap bean plants.
 Figure 2. Portable toilets with hand washing stations are to be placed within 1/4 mile of workers.
Figure 2. Portable toilets with hand washing stations are to be placed within 1/4 mile of workers.Regardless of the source, all irrigation water should be tested frequently for E. coli, which is an indicator of fecal contamination. While there are no set government standards for the quality of irrigation water, the California Leafy Greens Market Agreement for Best Practices (2007) has developed some guidelines, which were taken from EPA's recreational water standards for fresh water.
Acceptable E. coli levels for irrigation water have been set at less than or equal to 126 E. coli per 100 mL of water, where this figure is a geometric average of five water samples from each water source. This average value is valid for both foliar (overhead spray) and non-foliar (drip/furrow) irrigation applications.
Water sampling frequency calls for water testing to be done 60 days before harvest, with monthly testing during the production period. Many commodity growers are using this protocol for monitoring the safety of their irrigation waters. Growers should maintain records of all water tests with results and any corrective action taken. Inexpensive test kits for generic E. coli are available from several vendors. As an alternative, water samples can be sent to water testing or consulting labs to test for both E. coli levels and pesticide residues.
Frost Protection
Management of frost protection water is similar to that of irrigation water, or any water that makes direct vegetable contact. Ideally, water should be of drinking water quality (potable), if it comes in contact with the edible portion of vegetables.
Pesticide Usage
Since the water used to mix pesticides does come into direct contact with vegetables, this water should be from a potable source. Groundwater (from a well) should be given preference over surface water (from a lake or stream) because it has a lesser chance of becoming contaminated by direct or indirect contact with humans or animals. Municipal water is considered potable.
All pesticide usage should be done in strict accordance with manufacturer recommendations as well as federal, state and local ordinances. Monitoring and documentation of proper pesticide usage should be done to prevent unsafe or illegal residues from contaminating the beans. All pesticide applications should be documented; proper records of application should be available and reviewed by management on a regular basis. Appropriately trained and licensed individuals should perform all pesticide applications. Store pesticides in a locked location to prevent tampering.
Fertilizer Use
Improperly composted or un-composted (raw) manure is another source of human pathogens. Human pathogens may persist in animal manure for eight months. If compost is to be used on fresh snap beans, it must be certified that it has been completely treated so that no human pathogens are present.
"Fully composted" means that the organic matter has been maintained between 130° F and 170° F for a minimum of three days, using an in-vessel or static aerated pile system, or for 15 days using a windrow composting system, during which period the materials must be turned a minimum of five times (USDA National Organic Program, Final Rule, Section 205.203).
Animal manure (e.g., poultry litter) applied as a fertilizer must be incorporated into the soil at least 90 days prior to harvest, assuming the beans (bush-type) are not in contact with the soil. In contrast, animal manure applied as fertilizer must be incorporated into the soil at least 120 days prior to harvest, assuming that the beans (runner-type) may be in contact with the soil.
Composting manure, incorporating it prior to planting and avoiding top-dressing with fresh manure are steps that can be taken to reduce the risk of contamination while making use of this important source of nutrients. Composted sewage sludge should not be used as it may contain pathogens and heavy metal contaminants. Use of inorganic fertilizers that have been certified to be free of heavy metals and other chemical contaminants are always permitted.
Harvesting and Handling
Harvesting
Snap beans may be harvested by hand and packed directly in the field for the fresh market. They may also be harvested by machine, loaded into a bulk container or truck and hauled to a packing shed for cleaning, packaging and cooling. Field sanitation practices are very important to reduce the spread of disease among plants and to prevent the possibility of contamination by pathogens harmful to humans.
Field Worker Hygiene
During harvest operations, field workers may contaminate fresh snap beans simply by touching them with unclean hands or gloves. Portable field toilets equipped with hand wash stations must be available and used by all harvest crew members. Training, monitoring and enforcement of field worker hygiene practices, such as proper hand washing or glove washing procedures after using the toilet, are necessary to reduce the risk of human pathogen contamination. Once harvested, produce should not be placed on the soil before being placed into a clean and sanitary field container. If pickers are in the field for more than three hours, OSHA requires that there be one portable toilet per 20 employees. Toilet facilities must be movable, to be kept within one quarter mile (¼ mile) of the workers at all times. The sludge from the toilets must never be emptied onto the field or near surface water sources. A crew chief or supervisor must check toilets and hand wash stations frequently to ensure that they are well stocked with clean water, soap and single-use towels, and that proper hygiene procedures are being used.
Culling (Field-packed Beans)
Evidence suggests that human pathogens proliferate more readily in injured and decaying produce. This makes it important for vegetables showing bruises or decay symptoms to be culled as a preventative measure. Ideally, harvest workers should not handle culls in the field since this can spread infection from contaminated to healthy vegetables via the worker's hands or gloves. Culls should be removed by a separate worker ahead of the harvesting crew, if possible, so as to avoid contaminating good snap beans. These beans should be deposited in a separate "cull" bin and not left in the field. Color code the "cull" bin with paint so that it is not mixed in with the clean harvesting bins.
Field Container and Equipment Sanitation
Containers for field packing beans (picking buckets, wooden or fiberboard boxes) must be kept clean and in good repair. Picking buckets should be washed and sanitized at the end of each harvest day. Harvest containers such as wooden or fiberboard boxes must be inspected and found free of any extraneous material (e.g., protruding nail, staples, wood splinters, etc.) prior to use. During field packing, while picking buckets can be placed on the ground, harvest containers should not be allowed to touch the ground as this may transfer pathogens from the soil to the produce. Any commodity like snap beans that grows on or near the ground should have extra care taken to control soil contaminants, particularly at harvest time.
Machine harvested snap beans are very vulnerable to cross-contamination with human pathogens because sound beans are co-mingled with injured or decaying beans, soil and vines. Mechanical harvesters should be cleaned and adjusted daily. Fans should be set to remove the maximum amount of trash so it is left in the field. Bulk bins and/or truck body beds should be cleaned and sanitized frequently to keep disease inoculum from building on their surfaces and affecting sound snap beans.
Postharvest Handling
Receiving and Cleaning
Once harvested, field-packed boxes of snap beans must be immediately shaded from direct sunlight. This is to minimize heat build-up while waiting for transport to the packing shed. On arrival, beans must be rapidly cooled, preferably by forced air pressure cooling, to approximately 45° F. Human pathogens multiply slowly or not at all at this temperature.
Loads of machine-harvested beans are transported by truck to a packing shed where they are dumped into a large metal hopper, called a gravity separator, to allow rocks, soil and heavy field trash to drop out. Next, beans are conveyed to a trash eliminator, where air blasts remove leaves, stems, etc., and then to grading tables where workers manually remove defective (blemished, bruised, decayed, etc.) beans.
 Figure 3. After field trash is removed, beans are graded, then cooled in water flume.
Figure 3. After field trash is removed, beans are graded, then cooled in water flume. Figure 4. Free chlorine levels and pH in the flume wash water must be tested at least twice per shift during production.
Figure 4. Free chlorine levels and pH in the flume wash water must be tested at least twice per shift during production.
Hydro-cooling and Forced-air Cooling
Flume hydro-cooling is the fastest method of removing heat from snap beans, especially if they have been mechanically harvested and confined to a truck bed before transport to the packing shed. Cleaned and graded beans are deposited into an immersion-type flume system containing chilled (34-38° F) chlorinated water. Because water is a much better heat transfer medium than air, tests have demonstrated that uniform cooling will drop bean temperatures from 85° F to 45° F in about six minutes. Cooling is accelerated by the agitating motion of the water as beans flow through the flume. Rapid cooling not only removes field heat to maintain quality, but it helps to prevent brown end discoloration of the bean and slows or eliminates the multiplication of any human pathogens that might be present.
If flume hydro-cooling is not part of the packing line process, cleaned and graded fresh beans move to an automated filler where they are deposited into wirebound or waxed containers.
The alternative cooling method for boxed beans should be "forced air" pressure cooling. This method is superior to room cooling because it allows refrigerated air to be pulled through the beans to remove heat using high velocity fans. In contrast, room cooling does not have directed air flow to remove heat quickly; it merely sweeps across instead of through the boxes of beans.
Water Sanitation
Water used for cleaning and cooling snap beans at a packing shed must be potable (drinking quality) and sanitized. Chlorine is a popular sanitizer used in vegetable wash water systems. Chlorination can be accomplished by using a gas injection system, adding bleach or using calcium hypochlorite tablets to the water. Regardless of the form of chlorine added to the water, the active sanitizing agent is called free or available chlorine. The rationale for sanitizing snap bean flume water by adding chlorine is to keep the number of human pathogens from concentrating in the water and building up to an infectious dose, then cross-contaminating every snap bean that passes through the flume water. Research studies have demonstrated that the free chlorine concentration should be held between 100 and 150 ppm in order to achieve the desired sanitizing effect.
Water pH is also important. Studies have shown that when the pH of the flume water is controlled — ideally between 6.5 and 7.5 in pH — it will help keep free chlorine in its available form. Also, changing the flume water daily is important. The amount of free chlorine in the water decreases as the volume of organic matter (field debris) increases in the flume water. Therefore, a flume water change is necessary at least once a day minimum. A number of inexpensive chlorine test kits are on the market, but most test for the total chlorine content. However, a free chlorine test kit is required to perform the test for available chlorine in the wash water. Chlorination levels and pH of the flume water should be tested frequently during operation and recorded on a monitoring log or record.
Employee Health and Hygiene
 Figure 5. Bandage any wounds and protect the produce from potential contamination by wearing gloves.
Figure 5. Bandage any wounds and protect the produce from potential contamination by wearing gloves. Figure 6. Signs and sanitizer dispenser help to prevent contamination.
Figure 6. Signs and sanitizer dispenser help to prevent contamination.Good employee health and hygiene is critical to reducing food contamination by workers. It is important for supervisors to recognize symptoms of illness to keep sick workers away from the produce. Workers who display symptoms of illness should be assigned a job, if possible, where they do not have contact with the product or any equipment that will contact the product. Sending the worker home to recover is the best method of dealing with an ill employee.
Probably the number one source of foodborne disease is the unsanitary packing shed worker. It is for this reason that gloves, hairnets, clean smocks or aprons and boots (not to be worn outside) are common requirements for workers at a packing shed.
Most disease transmitted to fresh produce occurs by the "fecal-oral" pathway. This is the movement of human pathogens from an infected individual's waste to material ingested by a healthy person. Most commonly, this occurs when the infected individual handles produce without having properly washed his/her hands. Therefore, proper hand washing and toilet use are important steps in breaking the infection cycle. Conduct hand washing training for all new employees, in the language in which the employees are fluent, and document these training events as a permanent record to be kept on file.
Open wounds may also contain pathogens. The best method of reducing contamination from open sores or wounds is to use a bandage to protect the wound, followed by rubber or latex gloves on the hands to protect the produce. Depending on the situation, the worker might be transferred to another job so that they don't come into contact with the produce.
Remember, employees can maintain good hygiene only if proper facilities are available to them. Restrooms in packing sheds must include the following:
- A place to remove any aprons, smocks or gloves and hang them outside the restroom.
- Clean restrooms with liquid soap, clean water (with foot operated valves) and single-use, disposable towels or a hand-dryer system.
- A waste container for used towels.
- Modesty panels instead of doors on restrooms to avoid re-contaminating clean hands when opening the door. Posted signs in English, Spanish, etc., reminding workers to wash their hands before returning to work.
- A hand sanitizing solution or gel dispenser located outside the restroom, to be used in conjunction with, but not replacing, proper hand washing techniques.
Packing Shed Equipment and Facility Sanitation
Preliminary studies from USDA have demonstrated that unsanitary packing shed equipment may play a major role in contamination of fresh produce when produce-contact surfaces are not cleaned and sanitized satisfactorily. Therefore, produce contact surfaces such as the gravity separator, trash eliminator, flume tank, sorting and grading belts, etc., of a snap bean packing line should be cleaned and sanitized at the end of each production day.
Cleaning Equipment
First, spray equipment with an approved detergent cleaner using very low pressure to create foam that should loosen organic matter such as soil and plant material on contact surfaces. Rinse with potable water to remove the soil-foam complex. Next, a 200 ppm chlorine/bleach solution should be applied to sanitize bean contact surfaces. Avoid using steam or high pressure water sprays because they may create bacterial aerosols and actually help to spread contamination throughout the packing shed. Always start at the top of the equipment with any cleaning activity so that lower parts will not be re-contaminated.
Likewise, the packing shed facility has the potential for developing microbial growth (e.g., mold growth) on walls, ceilings, overhead pipes and fixtures, doors, drains, etc. Scheduled wash-down and sanitizing of the facility should be performed on non-production days (or nights) to control environmental contamination niches.
Cold Storage Facilities
Produce coolers — in particular, refrigeration coils and drip pans, forced-air cooling fans, drain tiles, walls and floors — are potential harbors for Listeria monocytogenes, an environmental pathogen harmful to humans. Unlike most pathogens, this bacterium can survive and even multiply under cold, moist conditions. It can contaminate produce if condensation from refrigeration units or the ceiling drips onto produce stored under these areas. Listeria monocytogenes is a normal inhabitant on fresh produce, so the only way to keep it from gaining a foothold in your cold storage facilities is to have a comprehensive scheduled cleaning and sanitizing of your coolers.
Packaging/Dry Storage
Since packaging materials come into direct contact with fresh produce, they may serve as a potential source of contamination. Packaging such as wooden wirebounds, waxed fiberboard boxes, plastic wrap and field bin totes should be stored in an enclosed area of the packing shed to prevent contact with rodents, insects, dust and dirt. Wooden and fiberboard boxes are impossible to adequately clean because they are too porous. Plastic surfaces such as RPCs are more amenable to cleaning and sanitizing, and should gradually replace wooden containers. If wooden field bins, boxes and fiberboard materials are used for packaging, line them with plastic to prevent the potential risk of cross contamination. Check stored units for contamination and either replace or clean any that have become soiled.
Pest Control
A pest control program will minimize risk of contamination by rodents or other animals. In an open or exposed packing house operation, the best control is constant vigilance and elimination of any discovered animals and their potential nesting locations (e.g., remove bird nests from rafters).
Product and/or product remnants will attract pests; therefore, the daily cleaning of the packing house to eliminate the attractive food sources should help to reduce pests. Be sure to have a licensed pest control operator (PCO) administer your pest control program.
 Figure 7. Loading pallets of green beans into cleaned trailer for transport to buyer.
Figure 7. Loading pallets of green beans into cleaned trailer for transport to buyer. Figure 8. Timely documentation of results is critical to auditors.
Figure 8. Timely documentation of results is critical to auditors.Transport Vehicles
Trucks must be inspected for sanitary conditions and optimum transit temperature before being loaded with fresh produce. Check for visible cleanliness, odors, dirt and/or other debris prior to loading. If the vehicle previously hauled raw meat, there is great potential for product contamination. Any truck showing these conditions should be rejected. Check for pest infestation, physical condition and the presence of a properly aligned air chute before loading. Make sure that a temperature recorder is present to monitor produce temperature during transit. Never load produce into a warm truck.
Traceback Plans
Recall of a food product is the last line of defense in a food safety emergency. Every produce grower, packer or shipper should develop and implement a recall or trace-back plan, and the organizational structure that will enable the removal of suspect product from the market in a rapid and efficient manner. Each produce container shipped from a farm or packing shed must have an identification code embossed or attached to the carton, box or container to allow management to trace this batch from the field of origin all the way to the distributor. The coded lot numbers should also be included on the bill of lading.
Record Keeping
Keeping records is very important. It will help to document adherence to good agricultural practices (GAPs) and to identify potential problem areas.
Keeping timely and accurate records can help to alleviate legal responsibilities in a trace-back situation.
Keep track of microbial test results, reefer and storage room temperature levels, employee training, etc.
History has shown that, in a trace-back situation following an outbreak, responsibility was often on those with the least (or worst-kept) records.
Self-check audit lists are available online from several commercial auditing companies, as well as the FDA, to aid in record keeping.
Remember — your records demonstrate due diligence and may prove that contamination did not originate in YOUR operation.
Harvest/Post Harvest and Waste Management
Dan MacLean, Horticulture
ChangYing Li, Biological and Agricultural Engineering
Gary L. Hawkins, Biological and Agricultural Engineering
Snap beans are immature fruit. Horticultural harvest maturity for snap bean is when the pods and seeds are both bright green in color. This usually occurs approximately one week to 10 days after flowering. Beans should be relatively straight and snap easily when bent. Beans should also be free from insect damage, malformed fruit and residual floral or leaf tissues. Immature beans appear thin and very dark in color while over-mature beans appear very plump and pale in color. Snap beans are hand harvested by pinching the stem slightly above the bean and pulling gently.
At the time of harvest, snap beans are growing very rapidly. As a result, they are a highly perishable product that should be cooled soon after harvest. The respiration rate of snap beans at 25° C (77° F) is six times greater than beans at 5° C (41° F). A 12-hour delay in cooling equates to a three-day loss of potential shelf life. Thus, due to the highly perishable nature of snap beans, every effort should be made to cool them as soon as practical after harvest in order to maximize their shelf-life and quality.
Snap beans should be cooled to 5-7.5° C (41-45° F) using a forced-air or hydro-cooler. If beans are harvested at low ambient temperatures, cooled as quickly as possible to the optimal storage temperature and maintained under high humidity, one can expect eight to 12 days of storage life. Controlled atmosphere (CA) conditions have been established for snap bean; however, commercial use of the technology is limited. The main benefit from CA is in color retention and minimizing discoloration losses due to harsh handling and bruising during harvest. Suitable CA conditions are O2 of 2 to 5 percent, and CO2 of 3 to 10 percent, all at 5-7.5° C (41-45° F) and high humidity (95 to 99 percent).
Ensuring a high relative humidity during the postharvest handling and storage period is critical for maintaining quality. Beans are very susceptible to desiccation and water loss is a common postharvest problem. Relative humidity should be maintained at 95 to 99 percent. Losses in quality, such as shrivel, will first appear after a 4 to 5 percent loss of moisture, and the beans will become unmarketable as water loss approaches 10 percent (by weight).
Chilling injury can occur within a few days at temperatures below 5° C (41° F) and appears as a diffuse non-localized discoloration of the entire bean. A different symptom of chilling, appearing as brown speckling on the surface, can appear at temperatures above 5° C (41° F) if stored for too long (more than 10 to 12 days). In general, no chilling injury is observed in beans stored at temperatures greater than 10° C (50° F); however, the postharvest storage life is greatly reduced under these conditions.
Ethylene is a common volatile produced by many ripening fruits and vegetables. Snap beans naturally produce low levels of ethylene but are considered moderately sensitive to the gaseous hormone. Presence of ethylene in snap bean can cause many undesirable effects, including discoloration, loss of green pigment and increased browning. Efforts should be made to ensure that storage and transportation of snap bean is separate from ethylene producing commodities, even if storage temperature recommendations are identical.
Most postharvest pathological disorders of snap bean are related to improper management during harvest and handling. Poor temperature management or inadequate cooling capacity may lead to moisture condensation on the surface of the bean during storage, resulting in surface decay in susceptible tissues. However, generally only beans exhibiting symptoms of chilling injury, broken or otherwise damaged beans result in significant pathogen nesting. Common decay organisms include Pythium and Sclerotinia, and generally occur at temperatures greater than 7.5° C (45° F).
When stored at temperatures between 68-70° F, snap bean has a high rate of heat respiration of 45,400-53,000 Btu per ton per day, which is even higher than strawberries and lettuce. Thus, it is particularly important for snap beans to be cooled rapidly after harvesting. Postharvest cooling quickly removes field heat from freshly harvested snap beans before shipment, storage or processing and is critical to ensure that quality is maintained until the snap bean reaches consumers. Proper postharvest cooling can suppress softening, slow or inhibit water loss, slow or inhibit the growth of decay-producing molds and bacteria, and reduce production of ethylene, a ripening agent. Under optimal conditions (40-45° F and 90 to 95 percent relative humidity), snap beans can be stored for seven to 10 days. In addition to protecting quality, postharvest cooling provides marketing flexibility by making it possible to market at the optimum time.
To choose an efficient and effective cooling system, there are many factors to consider, such as the nature of the produce and the final cooling costs, which include fixed costs (space, refrigeration equipment and air circulation system) and operating costs (refrigeration, air circulation and handling). Among the many cooling methods available, three are most suitable for snap beans: room cooling, forced-air cooling and hydro-cooling.
Room Cooling
Room cooling is the most conventional method for postharvest cooling. It is a cooling method that requires exposing snap beans to cold air in refrigerated space. A simple and effective arrangement is to discharge cold air into a cooling room horizontally just below the ceiling. The air sweeps the ceiling and returns to the cooling coils after circulating through the produce on the floor. Advantages of this simple cooling method include:
- snap beans can be cooled and stored in the same place, which requires less handling;
- design and operation are simple; and
- peak loads on the refrigeration system are lower than other, faster cooling methods.
However, room cooling also has several drawbacks:
- the cooling efficiency is low, particularly when containers have minimal open air spaces;
- this slow cooling may make the cooling inadequate in a given time, and sometimes snap beans may deteriorate in the time required to cool them; and
- it is difficult to control the cooling.
Forced-air Cooling
To preserve quality, snap beans should be cooled to their lowest safe (optimum) storage temperature as quickly as is practical and economical. Forced-air cooling is a rapid cooling method and four to 10 times faster than room cooling. It is used in conjunction with a cooling room and can be used effectively on most packaged produce. To increase the cooling rate, additional fans are used to pull cool air through the packages of produce. Although the cooling rate depends on the air temperature and the rate of airflow through the packages, this method is usually 75 to 90 percent faster than room cooling. When forced air cooling fans are added to an existing cooling room, it may be necessary to increase the size of the refrigeration unit to accommodate the additional cooling load. The fans are normally equipped with a thermostat that automatically shuts them off as soon as the desired produce temperature is reached to reduce energy consumption and water loss from the produce.
Compared to other cooling methods, the forced-air cooling method offers several advantages:
- Ensure better produce quality — It decreases the time the produce remains at elevated temperatures, thereby reducing deterioration;
- High cooling efficiency — It results in shorter cooling times and thus more efficient use of the cooling facility;
- Less handling and no wetting — It can cool produce effectively in a variety of unopened containers without wetting it or subjecting it to excessive handling;
- Energy efficient — It is often more energy efficient than room cooling when large volumes of produce must be cooled;
- Easy to retrofit — An existing room-cooling facility with adequate cooling capacity can be converted to forced air cooling with only a relatively small investment in fans and increase refrigeration.
Two different fan arrangements in the forced-air cooling system are proven to be successful and widely used in Georgia. The "shell arrangement" uses a portable pallet-mounted fan and is preferred because of its versatility. Two parallel rows of produce, positioned approximately 2 to 3 feet apart and covered by a cloth or plastic strip, form the shell. Cold air pulled through the produce flows through the space between the rows and out through the fan. Another fan arrangement, known as the "cooling wall," has the fans located permanently along one wall. This design might be more convenient for producers and shippers who handle large volumes of produce, especially if they always handle the same commodity or compatible commodities.
 Figure 1. Illustration of forced air cooling (cooling wall setup).
Figure 1. Illustration of forced air cooling (cooling wall setup).Hydrocooling
Hydrocooling is a cooling method that rapidly removes the heat from the produce by allowing chilled water to flow over the produce. At typical flow rates and temperature differences, water removes heat about 15 times faster than air. However, hydrocooling is only about 20 to 40 percent energy efficient, as compared to 70 or 80 percent for room and forced-air cooling. This cooling method can be used on most commodities that are not sensitive to wetting (wetting often facilitates the growth of microorganisms), including snap beans. Hydrocooling does not remove water from the produce, and actually may revive slightly wilted produce. Another advantage of this cooling method is that it can easily handle large amounts of produce, although hydrocoolers can be designed for smaller quantities as well.
When designing or using a hydrocooling system, there are several points to consider:
- water should move over the surface of the produce;
- water should contact as much of the surface of each fruit or vegetable as possible; and
- water must be kept as cold as possible without endangering produce.
 Figure 2. Illustration of a typical hydrocooler.
Figure 2. Illustration of a typical hydrocooler.The most common type of hydrocooler is the conveyor, which carries produce in bulk or in containers through a shower of water. Avoid the channeling effect by providing a heavy shower over a shallow depth of produce or by proportioning the shower and the drainage from the bottom of containers so that the containers fill partly or entirely with water.
The drawbacks of this cooling method include:
- hydrocooling can be used only for produce not sensitive to wetting (many diseases are encouraged by wetting);
- some hydrocoolers are not as energy efficient as other methods and therefore may not be as cost effective in some situations;
- there are some restrictions on the types of packaging and stacking arrangements used for produce that is to be hydrocooled.
Chlorinated water is usually recommended for washing and cooling snap beans during hydrocooling. Chlorine is a germicidal agent that is effective in controlling decay-causing organisms found on produce. A free chlorine concentration of about 55 to 70 ppm at pH 7.0 (neutral) is recommended to sanitize most fruits and vegetables. It may be necessary to add chlorine to the solution more often if the pH is higher and if the temperature of the solution is more than 80° F. There are some recommendations regarding the practice of chlorination:
- change the water frequently — chlorination efficiency is poor in very dirty water.
- avoid overexposure — do not allow the produce to remain in contact with the solution longer than necessary;
- dispose of waste water safely and with care — check all local, state and federal regulations for wastewater discharge.
- monitor the water condition — chlorine concentration and pH should be monitored frequently by the use of test papers or electronic equipment.
The snap bean is a perishable vegetable crop with a high rate of heat respiration. It is critical to remove the heat rapidly from snap beans after harvesting to preserve quality and extend shelf life. Three common cooling approaches — room cooling, forced-air cooling and hydrocooling — are available to cool snap beans. Growers and handlers should select the suitable cooling method with regard to the cost and efficiency.
Waste Management
Sorting and packing often results in waste material that is either rotten, has bad spots not noticed in the field or is not packed and shipped to the consumer. These snap beans that are removed from the sorting and packing lines are discarded. Properly dealing with discarded products can reduce the potential for environmental pollution while also protecting the originator of the discarded materials. Not all of the following methods of dealing with waste material may be applicable for all situations, but some portions could be used to deal with snap bean waste.
Managing Snap Bean Waste
There are seven commonly used methods of managing snap bean waste. The list of methods provided here will define the method of management, list some pros and cons of that method and discuss operational details. This list cannot be easily arranged in order of best management practice from an environmental standpoint due to individual situations of the farmer and packing house where culls originate. The management options are provided as a means to help better explain how each may be used. The seven management methods are:
- Store the culled snap beans on-site in a pile or bermed area for a limited time
- Return snap bean waste to the field on which it was grown
- Feed snap bean waste to livestock
- Give snap bean culls to local food banks
- Compost snap bean culls
- Process snap bean culls to separate juice from pulp
- Dispose of snap bean waste in a local Sub-Title D landfill
Managing Snap Bean Waste Based on Disposal Method
The following list of management methods provides a protocol for managing snap bean waste as well as pros and cons of each method.
1. Store the culled snap beans on-site.
Storing culled snap bean waste on-site is a temporary solution to final disposal or reuse of materials. The culls may be hauled or transferred via mechanical methods to a location that has been prepared for holding the culls. At a minimum, the holding area should be bermed to capture and hold rainfall and any liquids that have formed from the decomposition of the culled snap beans. Other options for such a site include storage in tanks or bunkers with easy access for removing liquids or solids for later management. The culls stored in the bermed area should be crushed, if possible, to allow available liquid to better evaporate. Crushing the culled snap beans and placing them in a bermed area helps control the leachate, run-on and runoff, makes managing the material easier, allows extra liquids to evaporate and reduces the volume that will need to be managed at a later time. The pros and cons of temporarily storing culls on-site are:
Pros:
- Low disposal cost
- Low transportation cost to disposal site based on reduced volume and distance
- Snap bean culls will generally decompose in weeks
- Associated snap bean juice will infiltrate into the ground
- A constructed bunker area will help improve solid and liquid management
- The same small footprint can be used year after year
Cons:
- Potential unsightliness for packing house and neighbors
- Potential odors associated with disposal of culled snap beans
- Tanks may need to be purchased, bunkers may need to be built and a method of crushing culls has to be in place
2. Return snap bean waste to the field.
From an agricultural nutrient management and organic building viewpoint, returning snap bean waste to the field may be one of the better options. This waste management method returns the culls back to the growing field where the nutrients can be recycled, allowing the snap bean pulp and juice to help build or maintain the soil organic matter content. The cost can be very low based on distance to the field and the amount of liquid removed. The protocol for transporting the culls to a growing field consists of storing the culls at the packing house or at the field site until final harvest of the crop. After final harvest, the culls or remaining solids and liquids can be loaded into spreader trucks and applied evenly across the field. As a matter of practice, the material should be incorporated, which will reduce the potential for problematic odors and runoff. The pros and cons of returning snap bean waste to the field are:
Pros:
- The nutrients in the snap bean culls can be available for next crop
- Organic matter in snap bean culls will increase soil carbon
- Low cost of disposal
Cons:
- Transferring snap bean culls from the trucks and trailers used to transport them back to the field site to the spreader equipment can be a problem
- High labor costs of unloading intact fruits and vegetables
- Trucks may have to be modified to carry and manage liquid resulting from crushed culls
- Bad snap beans can potentially result in disease transmission between harvested crops
- Proper distribution of culled fruits and vegetables can be a problem
- Snap bean culls cannot be returned to the growing field until all harvests (generally three to four per year) have occurred
- Snap bean culls have to be stored on-site until they can be disposed of in the field
3. Feed snap bean waste to livestock.
Managing culls by feeding snap bean waste to livestock maybe a good option based on the overall management system of the livestock operation. One of the major issues that must be addressed involves the nutritional benefits and effects of feeding culls to livestock. Farmers should consult with animal scientists or veterinarians to confirm the effects of feeding culls to livestock. If the nutritional aspect of feeding snap bean waste to livestock is met, other pros and cons include:
Pros:
- Low disposal cost
- Potential low transportation cost to livestock area
- Snap bean culls will potentially offset the cost of animal feed
- No need to wait until harvest is completed
- Snap bean culls can be taken to livestock at time of culling, thereby eliminating the need for storage.
- The sale of snap bean culls for feed can produce income
Cons:
- Livestock may not eat rotten snap bean culls
- Transportation cost may be high depending on distance to livestock area
- Volume of snap bean culls can be too high for available livestock to consume
- Incorporation of snap bean waste into animal diets may not improve animal productivity
- Removing snap bean culls from the trucks and trailers used to transport them back to the field site can be a problem due to the potential liquid content and methods for transferring to the spreader equipment
- High labor costs of unloading intact fruits and vegetables
4. Give good snap bean culls to local food banks.
Food banks may be an option to manage some of the culls resulting from the sorting of the fruits and vegetables. Giving culls to a food bank may be an option and the Good Samaritan Law will protect the donating company. However, since snap beans are perishable, not all of the culls can be utilized by this method. The farmer should stay in contact with the local food bank coordinator to inform them of harvest dates and what may be available, as well as to determine whether anyone would be allowed in the packing house and whether bins of culls would be available for further off-site culling and packaging for distribution to other food banks. The coordinator would need to provide a means to safely transport the culls to a location for further processing, if needed, and the remaining culls would have to be disposed using another method listed in this document. Some of the pros and cons of donating snap bean culls to a local food bank are:
Pros:
- Provides a needed food supply
- Low disposal cost
- The food bank may provide its own pick-up and delivery
Cons:
- Food banks can only use a portion of the snap bean culls
- Having food bank personnel on-site at packing may be a liability to the packing company
- The remaining portion of culled snap beans will still have to be disposed of through another appropriate method
5. Compost snap bean culls.
Composting culled fruits and vegetables is an option that can reduce the volume of culls as well as other "waste" materials in a community, if the land and equipment are available. Culls used in the compost process would either be transferred in a truck to the composting facility or mechanically transported if the compost facility is on-site. The culls would be mixed in proper ratios with other organic materials as recommended by composting professionals to produce compost suitable for reincorporation into fields or for selling. Georgia has a set of composting guidelines and regulations that should be referenced to ensure proper environmental protection and to obtain information on final management of the compost product. The pros and cons of a composting system are:
Pros:
- Low disposal cost
- Potential on-site composting
- Potential low transportation cost to disposal site
- Snap bean culls generally decompose in weeks
- Associated snap bean juice will be one source of needed water in the compost pile
- The final product can potentially be sold for profit
- The product can be returned to the growing field to provide stable nutrients and organic matter for the next crop
Cons:
- Filler material for composting snap bean culls can be a high cost
- Additional labor maybe needed to manage the compost pile properly
- Additional pest management maybe needed
- Runoff control will have to be implemented
- Getting rid of compost maybe a problem
- Proper permits have to be obtained
- Removing snap bean culls from the trucks and trailers used to transport them back to the field site can be a problem due to the potential liquid content and methods for transferring to the spreader equipment
- High labor costs of unloading intact fruits and vegetables
6. Process snap bean culls to separate juice from pulp.
The method of separating snap bean culls into juice and pulp is accomplished by using a press. Typical systems are screw presses that can effectively separate the juice from the pulp. After separation, each fraction has its benefits for different reasons and purposes. If the culls are of good food quality they can be used as juices in food applications based on available markets. The pulp can also potentially be used as a component of foods. For those culls that are not of human food quality, the separated pulp can be used as one component of compost or animal food. (If the pulp is used for animal feed, check with an animal scientist or veterinarian prior to feeding.) The pulp can also be used as a soil amendment or as one component of a composting process. The juice can also be used as a feedstock for ethanol production or anaerobic digestion processes. For either process, there should be a market for the final products, ethanol or methane. A few pros and cons of separating juice from pulp are:
Pros:
- Low disposal cost
- Potential on-site processing
- Low transportation cost to processing site
- Snap bean juice will evaporate quicker if separated from pulp
- Pulp can be used in composting
- Less composting filler material will be needed
- Pulp can be fed to animals
- Snap bean juice will be easier to transport and apply to the receiving field/pasture
- Snap bean juice can be a feedstock for ethanol or anaerobic digestion processes
- Snap bean juice is easier to store than whole snap beans.
Cons:
- A method of separating snap bean juice and pulp has to be in place
- Snap bean juice storage has to be in place
- Composting site and procedures have to be ready
- A tank is necessary for storing and transferring snap bean juice to the location to be used for the production of ethanol or anaerobic digestion
7. Dispose of snap bean waste in a local Sub-Title D landfill.
Disposal of culled snap bean waste in a Sub-Title D landfill is a method that should be considered after all other options. From a sustainability standpoint, disposal of these culls in a Sub-Title D landfill is probability not the best option based on fees. If landfilling is the chosen option, management of the culls should reduce leakage of liquids from the transport truck. The following are pros and cons of landfilling culls:
Pros:
- Once the snap bean culls are dumped, all responsibility is transferred to landfill operator/owner
- Juice associated with snap bean decomposition could increase methane production in the landfill that would be beneficial if the landfill is collecting methane for energy production
Cons:
- High cost of disposal in tipping fees
- Potentially high transportation cost to disposal site
- Juice associated with snap bean culls will add to leachate that has to be handled by landfill operator/owner
- Juice associated with snap bean decomposition could increase methane production in landfill
- Juice from decomposing snap beans that can drain out of a truck/trailer transporting snap bean culls to the landfill must be accounted for and managed
- If leakage from transport trucks occurs, complaints, bad press and regulatory issues could be possible
Management of Snap Bean Waste for a Specific Packing House
The above methods for the disposal or reuse of snap bean waste are provided for all snap bean packing houses. The disposal method specific for any given packing house may be different and must be a decision based upon the particular location and situation. There may be more disposal methods available to specific packing houses, additional pros and cons not listed here and different and more extensive methods of disposing the culled snap beans. Individual packing houses will have to identify additional pros and cons and take them into account. This document is provided only as a guide to aid the individual packing house in identifying different options that may be suitable for disposing of culled fruits and vegetables. Detailed questions related to local and state regulations and ordnances should be directed to local and state agencies that specifically deal with those questions. For application rates to meet nutrient needs of fields or growing crops, contact your local county Extension office.
References
AG-413-8, Postharvest Cooling and Handling of Green Beans and Field Peas. North Carolina State University.
AG-414-3, Forced Air Cooling. North Carolina State University.
AG-414-4, Hydrocooling. North Carolina State University.
Marketing
Greg Fonsah, Extension Agricultural Economist
Introduction
Snap beans are an important horticultural crop for the state of Georgia in particular and the U.S. at large. In 2007, the Georgia Farm Gate Value Report showed that snap beans ranked third in acreage and eighth in the value of vegetables (Table 1). In 2008, this crop ranked 41st in Georgia Agricultural Commodity (Boatright and McKissick, 2008; 2009). Marketing snap beans or any horticultural product is about more than just selling. Marketing includes planning, production, harvesting, packaging, transportation, distribution, warehousing and pricing. It also means being in compliance with the health and safety regulations created by consumers and imposed by various regulatory agencies.
To be successful, marketing must be customer oriented and responsive to consumers' demands. Producers today need marketing skills and knowledge of the targeted market, whether it is direct marketing, marketing to retail outlets, specialty food stores or wholesalers. Questions to keep in mind include: Do you need any promotion? Is any specific harvest time required? Do you already have a customer/purchaser? Do consumers demand quality, freshness, "reasonable" prices or all of the above?
| Table 1. Top Ten Georgia Vegetable Acreage and Value Ranks, 2007 | |||||
| Rank | Crop | Total Acres | Rank | Crop | Total Acres |
| 1 | Sweet Corn | 25,816 | 1 | Onions | $164,391,978 |
| 2 | Watermelon | 24,215 | 2 | Watermelon | $104,604,248 |
| 3 | Snap Beans | 20,355 | 3 | Bell Peppers | $100,131,847 |
| 4 | Onions | 13,839 | 4 | Sweet Corn | $ 93,785,132 |
| 5 | Cucumbers | 10,655 | 5 | Cucumbers | $ 86,345,401 |
| 6 | Cabbage | 10,424 | 6 | Tomato | $ 49,658,267 |
| 7 | Collards | 7,329 | 7 | Cabbage | $ 40,116,806 |
| 8 | Turnip Greens | 7,094 | 8 | Snap Beans | $ 29,424,918 |
| 9 | Bell Peppers | 5,875 | 9 | Yellow Squash | $ 25,153,430 |
| 10 | Yellow Squash | 5,727 | 10 | Cantaloupe | $ 23,806,672 |
| Total Farm Gate | |||||
Area Planted and Harvested
The production of snap beans in Georgia has been increasing steadily since 1998 when 13,000 acres were planted and 12,000 acres were harvested. In 2008, 19,000 acres were planted and 17,000 acres were harvested (Figure 1). During this decade, we experienced two peak productions — in 2004 and 2006 — when planted area rose to 20,000 acres, although 17,000 acres were harvested in 2004 compared to 19,000 acres in 2006. The increase in production and harvested area may be associated with many factors, including improvement in research and extension activities, improved varieties, better control of pests and diseases, growers' understanding and use of improved technologies and good agricultural practices.
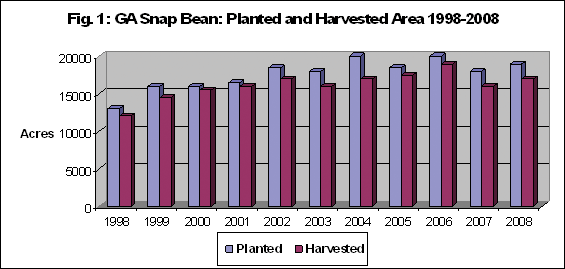 Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Yield Trend
For the past decade, snap bean yield trends have been fluctuating. For instance, there was a slight increase in the 1998 yield of 52 cwt per acre compared to 55 cwt per acre in 2008. The highest yields were recorded in 2000 and 2001 when 56 cwt and 60 cwt per acre was produced, respectively. The worse years were 1999, 2002, 2005 and 2007, when yields were below or slightly below 41 cwt per acre (Figure 2). Yield volatility can be caused by several factors ranging from pest and disease problems to hurricanes and tropical storms.
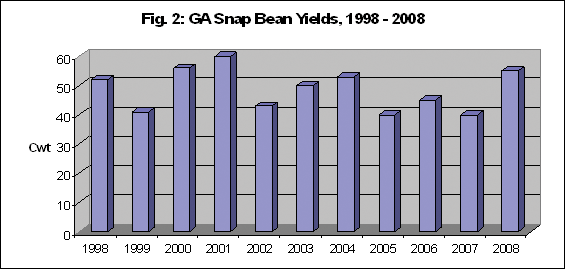 Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia
Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Production
Like yield trends, total production trends in Georgia have fluctuated from year to year; however, in the past 10 years it has risen overall. The lowest production was in 1999 when 595,000 pounds per acre was recorded and the highest production was in 2001 when 960,000 pounds per acre was recorded. Year 2008 was also a good year even though production fell slightly to 935,000 pounds per acre (Figure 3). Other good years for Georgia were 2000, 2004 and 2006.
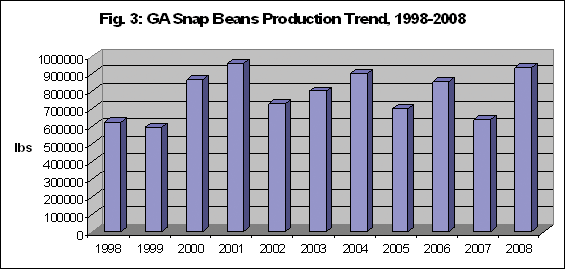 Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Average Seasonal Price
The Georgia snap bean industry has enjoyed favorable prices in the past years. Excellent prices were obtained in 2000, 2003 and 2006 when $28, $30 and $29 per cwt were recorded, respectively, (Figure 4). The lowest price was in 2001 when the price fell to barely $22 per pound. The reason for this drastic fall in price was due to the significant increase in production in 2000 and 2001 (Figure 3).
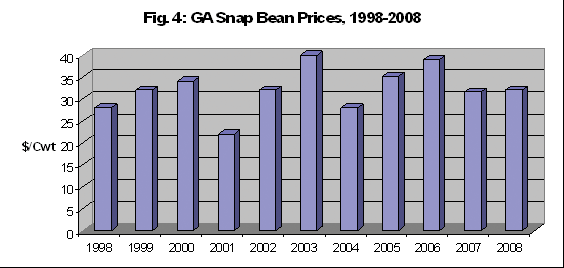 Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Farm Gate Value
Although the Georgia snap bean farm gate value has been impressive, there were four exceptional years when the value was above $25 million. The farm gate values for 2000, 2003, 2006 and 2008 were $29,512, $32,000, $33,345 and $29,920, respectively. The highest value was $32,000 in 2006 compared to the lowest value of $17,472 in 1998 (Figure 5).
Decreased farm gate values in 1998 and 1999 were caused by the decrease in production in the same time period. Even though prices were relatively higher, the high prices were not enough to offset the impact of the decrease in production.
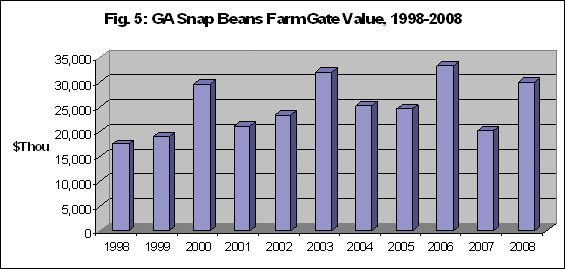 Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Production and Seasonal Average Price
For the past decade, snap bean production has been consistently increasing, with a few fluctuations in some years. Besides 1999 when less than 600,000 produced were produced, the subsequent years have been relatively higher. In 2000, 2001, 2004, 2006 and 2008, production was at record highs. The highest was in 2001 when a total of 960,000 pounds of snap beans were produced. In 2008, we came close to that record after producing 935,000 pounds (Figure 6).
However, it is interesting to note how prices respond to production. In 1998 and 1999 when production was relatively lower, seasonal average price steadily increased. Then as production picked up from 2000 and 2001, a drastic fall in price occurred — in fact, the lowest in the 10-year span occurred at $22 per hundred weight (cwt). Similar production and price trends were recorded in 2002 and 2003. Overall, the peak price of $40 per hundred weight (cwt) occurred in 2003 and the relative peak in 2006 was $39 per hundred weight (cwt).
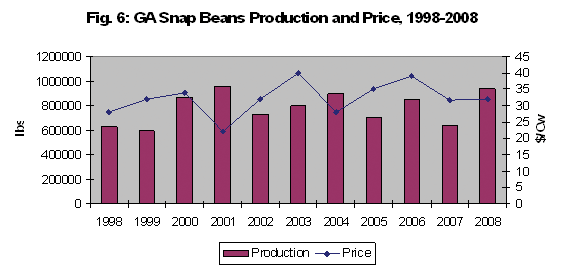 Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Source: Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
U.S. Monthly and Average Seasonal Price Trend
The United States price trend for snap beans shows that seasonal prices steadily increased from 1998 to 2008 (Table 2). The highest price of $60.50 per hundred weight (cwt) was recorded in 2007, while the lowest price was recorded in 2000. Monthly prices fluctuated significantly. In March 2007, price per cwt rose to a peak of $102; the lowest price of $35.10 per cwt occurred in June. In the fall production of the same year, the highest price per cwt was $89.30 in November. It is important to note that price alone is not enough to determine profit margin. Cost of production varies from state to state and from region to region, and has a significant impact on profitability (Table 2).
Table 2. U.S. Monthly and Average Seasonal Price of Snap Beans, 1998-2008
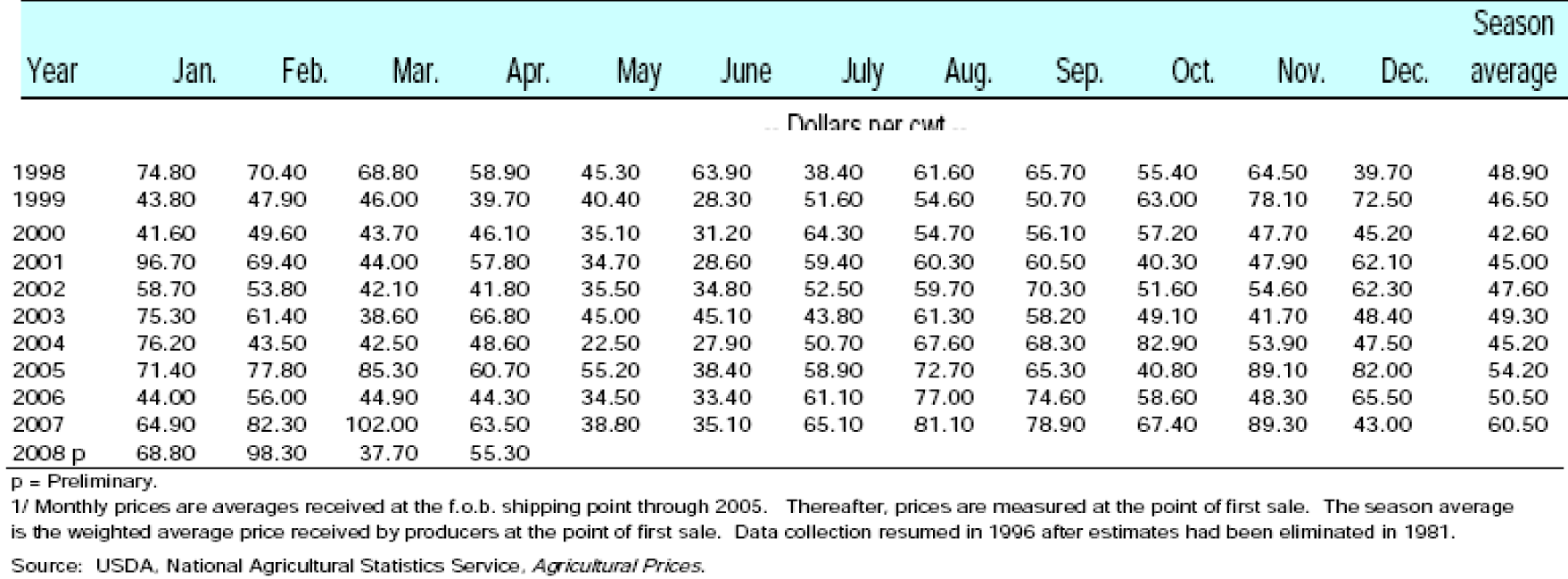
World Acreage
The United States is ranked first in terms of snap bean acreage. In 1997, 1998, 2001, 2003, 2004 and 2006 the United States harvested slightly below 300,000 acres. On the other hand, in 1999, 2000, 2002 and 2005, more than 300,000 acres were harvested. Overall, the United States provides more than 40 percent of the total world snap bean acreage. Mexico and France are ranked second and third, respectively. Mexico's acreage was almost 28 percent of the world total (Table 3).
Table 3. World Harvested Acreage of Snap Bean, 1997-2006
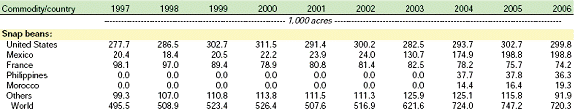 Source: Vegetable and Melons Situation and Outlook Yearbook/VGS-2008/ May 15, 2008.
Source: Vegetable and Melons Situation and Outlook Yearbook/VGS-2008/ May 15, 2008.
World Production
The United States is also the number one producer of snap beans in the world. In 1997 and 1998 the U.S. produced more than 20 million cwt per year. In 2006, the U.S. produced 38.4 percent of the world's snap beans, at 57.5 million cwt (Lucier and Dettmann, 2008). Mexico and France contributed 16.0 million cwt and 7.8 million cwt, respectively, in 2006, which translates to 27.8 percent and 13.6 percent of the world total, respectively. Overall, the world production was at its peak in 2004 with a total of 58.0 million cwt. Other important snap bean-producing countries are Morocco and the Philippines (Table 4).
Table 4. World Production of Snap Bean, 1997-2006 (Million Cwt)
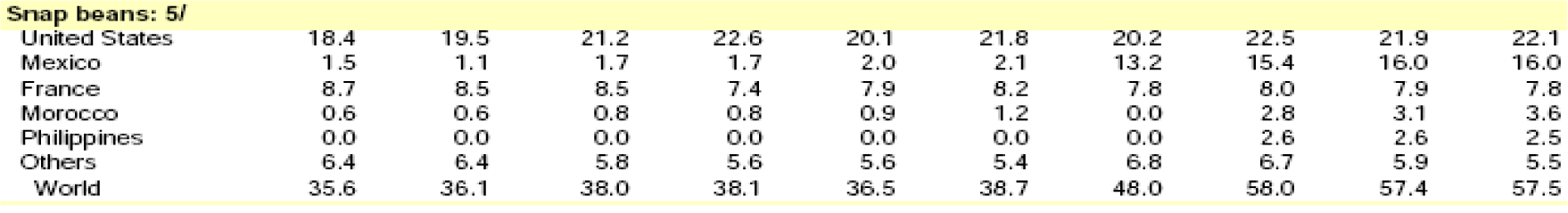 Source: Vegetable and Melons Situation and Outlook Yearbook/VGS-2008/ May 15, 2008.
Source: Vegetable and Melons Situation and Outlook Yearbook/VGS-2008/ May 15, 2008.
Import Trade
Despite the fact that the United States is the largest producer of snap beans, import trade has steadily increased. From 1990 to 1998, the quantity imported fluctuated between 30 million pounds and 45 million pounds. From 2000 to 2008, significant growth in imports moved from 49 million pounds to 70 million pounds, which is an increase of about 42.9 percent (Table 5).
Table 5. United States Monthly and Annual Snap Beans Imports, 1998-2008
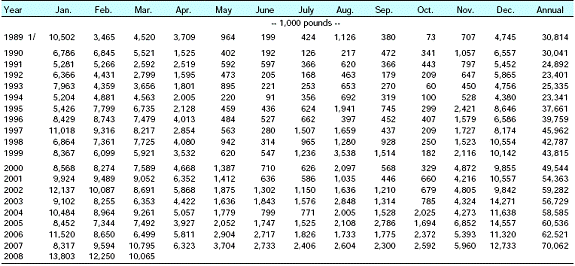 Import data beginning in January 1989 are reported under the harmonized tariff schedule (HS). U.S. Dept of Commerce. (Source: Vegetable and Melons Situation and Outlook Yearbook/VGS-2008/ May 15, 2008.)
Import data beginning in January 1989 are reported under the harmonized tariff schedule (HS). U.S. Dept of Commerce. (Source: Vegetable and Melons Situation and Outlook Yearbook/VGS-2008/ May 15, 2008.)
Export Trade
Although the United States is ranked first in produced and harvested acreage of snap beans, only a small quantity is exported. Table 6 depicts Canada as the number one importer of U.S. snap beans, followed by Mexico. Other countries that import snap beans from the U.S. are the United Kingdom, the Netherlands, Germany, South Korea, Hong Kong China, Taiwan and Japan (Table 6). In 2007, U.S. export value for snap beans was more than $40 million (Lucier and Dettmann, 2008; Fonsah, 2006b).
Table 6. U.S. Export Value of Fresh Vegetables to Selected Countries and the World, 2007
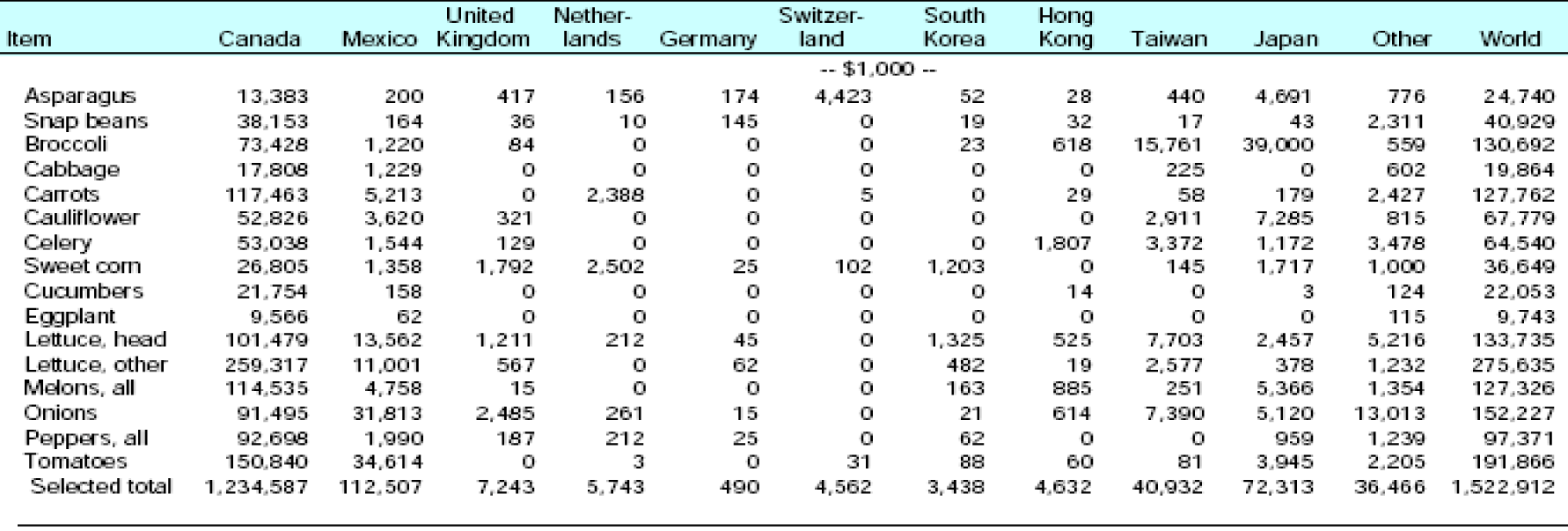 Source: Vegetable and Melons Situation and Outlook Yearbook/VGS-2008/ May 15, 2008
Source: Vegetable and Melons Situation and Outlook Yearbook/VGS-2008/ May 15, 2008
Retail Prices
The U.S. average retail price for snap beans in January 2008 was higher than 2007 and 2009. The peak retail price in 2007 occurred in March and drastically down-trended thereafter until August when there was a switch from a downward to an upward trend. A relative peak was observed in November. In 2008, the industry suffered a significant fall in retail price in February. A relatively higher retail price was seen from July to September of the same year (Figure 7). Several reasons could explain these fluctuating prices, such as excessive domestic supply and excessive import supply (USDA/NASS, 2009; Fonsah, 2006a; 2006b).
 Figure 7. U.S. Average Snap Beans Retail Prices By Month: 2007-2009. Source: USDA, NASS, Agricultural Prices. Accessed May 15, 2009. .
Figure 7. U.S. Average Snap Beans Retail Prices By Month: 2007-2009. Source: USDA, NASS, Agricultural Prices. Accessed May 15, 2009. .
References
Boatright, S.R. and C. McKissick. 2007 Georgia Farm Gate Value Report, AR 08-02, ½ûÂþÌìÌÃ, College of Agricultural and Environmental Sciences, Center for Agribusiness and Economic Development.
Boatright, S.R. and C. McKissick. 2009 Georgia Farm Gate Value Report, AR 09-01, ½ûÂþÌìÌÃ, College of Agricultural and Environmental Sciences, Center for Agribusiness and Economic Development.
Fonsah, E.G. (2006a). "Marketing" In: E.G. Fonsah and A.N. Sparks (Eds). Commercial Pepper Production Handbook. University of Georgia Cooperative Extension Bulletin 1309, p. 47-50.
Fonsah, E.G. (2006b). "Marketing" In: A.N. Sparks and E.G. Fonsah (Eds). Commercial Tomato Production Handbook. University of Georgia Cooperative Extension Bulletin 1312 p. 42-47.
Georgia Agricultural Statistics Service/USDA, 2002 Census of Agriculture Georgia Profile. www.nass.usda.gov/QuickStats/PullData_US.jsp. Accessed May 15, 2009.
Lucier, G. and R.L. Dettmann (2008). Vegetable and Melons Situation and Outlook Yearbook/VGS-2008/ May 15, 2008. ERS/USDA. Accessed: 07/28/2009.
USDA, NASS, Agricultural Prices. . Accessed May 15, 2009.
Production Costs
Greg Fonsah, Extension Agricultural Economist
Production Costs
Estimating cost of production is an important aspect of risk management. It is absolutely important for growers of any commercial vegetable to know whether their enterprise is profitable or not. This information is pertinent to growers for sound decision making. This aspect of risk management is also applicable to snap bean growers who can equally use enterprise budgets to determine the lucrativeness of their business. As a rule of thumb, an enterprise budget includes cost estimates for those inputs necessary to achieve the specified snap bean yields during cultivation years. Since production practices vary among growers, each grower needs to adapt budget estimates to reflect his or her individual situation (Fonsah and Hudgins, 2007; Fonsah et al. 2007; Fonsah, 2008; Fonsah et al, 2008). Detailed printed and computerized budgets are available in most county Extension offices and on the University of Georgia Department of Agricultural and Applied Economics Web site: http://www.ces.uga.edu/Agriculture/agecon/printedbudgets.htm
Types of Costs
There are several costs involved in producing snap beans. Economists classify these costs into variable and fixed costs. Another word commonly used for variable cost is operating cost. The variable or operating costs vary with the adopted snap bean cultural practices. Some variable cost inputs include seed, fertilizer, chemicals, fuel and labor. Variable costs are usually broken down into pre-harvest, harvesting and marketing operations in the budget. This break-down provides the grower an opportunity to review his/her production costs at different stages of the operation and production process (Fonsah et al., 2007; Fonsah et al., 2008; and Byrd et. al., 2006). For instance, Table 1 shows that total pre-harvest or pre-variable or pre-harvesting cost was $916, cost of seeds was $250, fertilizer application including lime and side-dressing was $151, and labor and irrigation were both $96 each. Land cost may either be a variable or a fixed cost. Since it varies significantly from county to county and from region to region, and depends on whether it is irrigated or non-irrigated, it is not included in this budget. Land owners still accrue a cost associated with their land.
| Table 1. Variable or Operating Costs of Producing nap Beans in Georgia, 2009 | ||||||
| Item | Unit | Quant | Price | Amt/acre | Total | Yours |
| Pre-Harvest Costs | ||||||
| Seed | Lb. | 50.00 | 5.00 | 250.00 | 250 | |
| Lime, applied* | Ton | 0.50 | 40.00 | 20.00 | 20 | |
| Fertilizer, 5-10-15* | Cwt | 6.00 | 17.00 | 102.00 | 102 | |
| Sidedressing, 19% N* | lbs | 60.00 | 0.48 | 28.80 | 29 | |
| Insecticide | Appl. | 4.50 | 13.10 | 58.95 | 59 | |
| Fungicide | Appl. | 4.00 | 13.00 | 52.00 | 52 | |
| Nematicide | Acre | 3.00 | 10.50 | 31.50 | 32 | |
| Herbicide | Acre | 1.00 | 25.00 | 25.00 | 25 | |
| Machinery | Acre | 1.00 | 110.34 | 110.34 | 110 | |
| Repair and Maintenance | Acre | 1.00 | 25.00 | 25.00 | 25 | |
| Labor | Hrs | 12.00 | 8.00 | 96.00 | 96 | |
| Land rent | Acre | 1.00 | 0.00 | 0.00 | 0 | |
| Irrigation | Acre | 12.00 | 8.00 | 96.00 | 96 | |
| Interest on Operation. Cap. | $ | 895.59 | 0.09 | 20.15 | 20 | |
| Total Pre-Harvest or Pre-Variable Cost | 915.74 | 916 | ||||
| * Fertilizer amount and application rates should be based on recommendations. | ||||||
Harvesting and Marketing Costs
Harvesting and marketing costs include picking and hauling, grading and packing, and packaging and marketing. The cost in the example was computed based on the average yield of 170 bushels per acre, which it is assumed the grower can obtain 50 percent of the time (Fonsah et al., 2007; Fonsah et al., 2008; and Fonsah and Hudgins, 2007). The total harvesting and marketing cost was $1,292. Picking and hauling 170 bushels at the cost of $1.50/bushel was $255, while shelling and packing at $2.20/bushel was $374 per acre. Other important cost components were containers and marketing. The total harvest and marketing cost was $1,292. The total variable/operating cost (i.e., pre-harvest plus harvesting and marketing costs) was $2,208 (Table 2). The total variable or operating cost in Table 2 is the sum of the total pre-harvesting and/or pre-variable costs plus total harvesting and marketing costs.
| Table 2. Harvesting and Marketing Costs of Producing Snap Beans in Georgia, 2009. | ||||||
| Item | Unit | Quant | Price | Amt/acre | Total | Yours |
| Harvesting and Marketing Costs | ||||||
| Picking and hauling | Bu. | 170 | 1.50 | 255.00 | 255 | |
| Shelling and packing | Bu. | 170 | 2.20 | 374.00 | 374 | |
| Container | Bu. | 170 | 1.90 | 323.00 | 323 | |
| Marketing | Bu. | 170 | 2.00 | 340.00 | 340 | |
| Total Harvest and Marketing | 7.60 | 1292.00 | 1,292 | |||
| Total Variable/Operating Costs | 2207.74 | 2,208 | ||||
Fixed Costs
The fixed costs of cultivating snap beans include items such as equipment ownership (depreciation, interest, insurance and taxes), management and general overhead costs. Overhead and management cost reflect 15 percent of total pre-harvesting variable or operation costs, which cannot be allocated to any one specific enterprise. Overhead items include utilities, pick-up trucks, farm shop and equipment, and fees. Most of these costs are incurred even if little production takes place and should be considered when planning production costs (Fonsah and Hudgins, 2007; Fonsah et al., 2007; and Fonsah et. al., 2008; Bryd et al., 2006). The total fixed cost, which is the sum of machinery, irrigation, overhead and management, was $326. Here the irrigation cost does not include the cost of running the system on a daily basis, as that is part of the variable cost. Table 3 shows a machinery cost of $89, which includes tractor, plow, disc, herbicide applicator, planter, cultivator and sprayer. The calculation also includes salvage value, equipment life span, depreciation, interest, taxes and insurance.
| Table 3. Fixed Costs of Producing Fresh Snap Beans in Georgia, 2009. | ||||||
| Item | Unit | Quant | Price | Amt/acre | Total | Yours |
| Machinery | Acre | 1.00 | 88.61 | 88.61 | 89 | |
| Irrigation | Acre | 1.00 | 100.00 | 100.00 | 100 | |
| Overhead and Management | $ | 915.74 | 0.15 | 137.36 | 137 | |
| Total Fixed Costs | 325.97 | 326 | ||||
| Total Budgeted Cost Per Acre | 2533.72 | 2,534 | ||||
Break-Even Cost of Production
The break-even (BE) cost of production (Table 4) is broken down in cost per unit. The BE pre-harvesting variable costs and the fixed costs of producing snap beans decline with increasing yields. The BE pre-harvesting variable cost per bushel of producing snap beans in Georgia was $5.39, whereas the BE harvesting and marketing cost per bushel was $7.60. On the other hand, the BE total budgeted cost per bushel was $14.91.
| Table 4. Break-Even Costs/Bushel of Producing Snap Beans in Georgia, 2009 | |
| Costs Per Bushel | |
| BE pre-harvesting variable cost per bushel | $5.39 |
| BE harvesting and marketing cost per bushel | $7.60 |
| BE fixed costs per bushel | $1.92 |
| BE total budgeted cost per bushel | $14.91 |
Risk Rated Net Returns
Snap bean yields and prices fluctuate significantly. Several factors, such as adopted agricultural practices, pest and disease problems, draught, torrential rains, excess supply or shortages can be blamed for these differences. Due to variation in yields and prices from year to year, an attempt was made to estimate the riskiness of producing snap beans in Georgia. The Extension Agricultural Economics Department uses five different yields and prices to calculate risk. The median values are those prices and yields that a particular snap bean grower would anticipate to exceed half the time (Fonsah and Hudgins, 2007; Fonsah et al., 2007 and Fonsah et. al., 2008) (Table 5).
| Table 5. Risk Rated Return of Yields and Prices of Snap Beans in Georgia, 2009. | |||||
| Snap Beans, Fresh Market — Total Cost Budget Number of acres 1 |
|||||
| Best | Optimistic | Median | Pessimistic | Worst | |
| Yield (bushels) | 210 | 190 | 170 | 150 | 130 |
| Price per bushel | 23.00 | 21.00 | 19.00 | 17.00 | 15.00 |
In Table 5, the median yield was 170 bushels/acre. The best was 210 bushels and the worst was 130 bushels/acre. On the other hand, the median price was $19.00/bushel while the best and worst prices were $23.00 and $15.00 bushel/acre. Half the time, a snap bean grower would anticipate not reaching below these prices and yields. On the other hand, both optimistic and pessimistic values are those prices and yields snap bean growers would expect to reach, exceed or fall short of one year in six. The best and worst values are those extreme levels that would occur once a lifetime (one year in 49 years).
Risk-Rated Returns Over Total Costs
The risk-rated returns over total costs shows that 7 percent of the time growers can optimistically obtain a net return of up to $1,310 and pessimistically as low as $82 per acre. This risk-rated return shows that 50 percent of the time the net revenue will be $696 with a 96 percent chance of making a profit (Table 6).
Budget Use
An enterprise budget has multifaceted uses, not just estimating the total costs and break-even costs for producing snap beans. The estimation of cash costs (out-of-pocket expenses) is important as it reveals how much money is needed to successfully carry out the project from start to finish from the both the agronomic and marketing perspective. Moreover, it provides information on how much a grower needs to borrow from a financial institution. It is also important as it assists growers in preparing cash flow statements.
| Table 6. Snap Bean Production Risk Rated Returns Over Total Costs in Georgia, 2009. | |||||||
| Net return levels (TOP ROW); | |||||||
| The chances of obtaining this level or more (MIDDLE ROW); | |||||||
| The chances of obtaining this level or less (BOTTOM ROW). | |||||||
| Optimistic | Expected | Pessimistic | |||||
| Returns ($) | 1,310 | 1,106 | 901 | 696 | 492 | 287 | 82 |
| Chances | 7% | 16% | 31% | 50% | |||
| Chances | 50% | 31% | 16% | 7% | |||
| Chances for Profit | 96% | Net Revenue | 696 | ||||
Conclusion
Estimating cost of production is an important aspect of risk management. It is very important for growers of any commercial vegetables to know whether their enterprise is profitable or not. This information is pertinent to growers for sound decision making. This aspect of risk management is also applicable to snap bean growers who can use enterprise budgets to determine the lucrativeness of their business. A risk-rated enterprise budget depicts that a grower in Georgia may earn a net return of $1,310/acre of snap beans 7 percent of the time, $1.106/acre 16 percent of the time and as low as $696/acre 50 percent of the time. Also, this study reveals that there is a 96 percent chance of obtaining a profitable margin in snap bean production.
References
Byrd, M.M., C.L. Escalante, E.G. Fonsah, and M.E. Wetzstein (2006). "Financial Efficiency of Methyl Bromide Alternatives for Georgia" Bell Pepper Industries. Journal of the American Society of Farm Managers and Rural Appraisers, 69 (1) 31-39.
Fonsah, E.G. (2008). "Production Cost of Onions In: Onion Production Guide, eds. G.E. Boyhan and W.T. Kelley. University of Georgia Cooperative Extension Bulletin 1198-2, p. 49-52.
Fonsah, E.G., G. Krewer, K. Harrison and D. Stanaland (2008). "Economic Returns Using Risk Rated Budget Analysis for Rabbiteye Blueberries in Georgia," Journal of American Society for Horticultural Science, HortTechnology, July-September; 18: 506-515.
Fonsah, E.G. and J. Hudgins (2007), "Financial and Economic Analysis of Producing Commercial Tomatoes in the Southeast," Journal of the American Society of Farm Managers and Rural Appraisers 70 (1): 141-148.
Fonsah, E.G., G. Krewer, K. Harrison and M. Bruorton (2007). "Risk Rated Economic Returns Analysis for Southern Highbush Blueberries in Soil in Georgia". Journal of American Society for Horticultural Science, HortTechnology 17 (4): 571-579 (Oct-Dec).
Organic Production
George Boyhan, Extension Horticulturist - Vegetables
Green beans can be grown under organic restraints with ease. In fact, they can be an important part of an organic farm plan because they are legumes. Legumes, through bacteria that colonize their roots, are capable of fixing atmospheric nitrogen. This can be an important source of nitrogen and part of an overall soil fertility program.
, even though it gives recommendations in inorganic fertilizers, can be an important part of planning your fertility program. Refer to ½ûÂþÌìÌà Cooperative Extension Circular 853 "How to Convert an Inorganic Fertilizer Recommendation to an Organic One" to see how to use this information.
For information on potential diseases and insects that may affect snap beans, refer to the Insect and Disease sections elsewhere in this publication. Under certified organic production, the use of inorganic chemicals to control these potential problems is generally prohibited. There are many cultural and management strategies that can be employed to help control these problems. For a general discussion on organic production and managing insects and diseases, see ½ûÂþÌìÌà Cooperative Extension Bulletin 1300, "Commercial Organic Vegetable Production."
Probably the most difficult problem to manage under organic restraints is weeds, and a variety of management strategies should be employed. These could include cover crops, soil solarization, stale seedbed, cultivation, plant spacing and mulches. Not all weed control methods will be completely effective; therefore, a number of methods may be necessary. Some weeds may be particularly difficult or impossible to control, such as nutsedge or bermudagrass, in which case a different location may be advised.
The organic market is particularly interested in different or unusual food sources. Wax beans, although not unusual, are yellow versions of snap beans and can be produced with the same cultural practices. There are many legumes related to snap beans that may be of interest to organic growers. Edamame are immature soybean pods that are boiled, usually with salt, and served whole. Many seed companies offer varieties specifically for edamame. A winter legume, fava beans, may also be of interest to organic growers, particularly because it offers a product when fresh snap beans are not available.
Organic growers are more apt to save and share seed. Beans are an easy to save and store seed, and since virtually all are open-pollinated, they will come true-to-type. Growers should exercise caution when getting seed from unknown sources. There are legumes that can be poisonous. In addition, several edible beans require proper preparation before consumption. This usually involves bringing to a boil for several minutes. In some cases the water should be poured off prior to consumption.
For specific information on certified organic production, refer to the National Organic Program (NOP) or your local county Extension agent. The rules for certified production and a list of certifying agents are available from the USDA NOP Web site ().
Status and Revision History
Published on Jul 27, 2010
Published with Full Review on Jul 01, 2013
