- Prevalence of Mastitis in Unbred and Pregnant Heifers
- Mammary Leukocyte Response to Intramammary Infection
- Mammary Tissue Response to Presence of Infection
- Use of Antibiotic Therapy to Control Mammary Infections
- Managing Heifers to Prevent Mammary Infections
- Summary
- References
Replacement heifers are critical to dairy herd productivity because they represent the future milking and breeding stock of all dairy operations. The goal should be to provide an environment for heifers to develop their full lactation potential at the desired age with minimal expense. Animal health and wellbeing play vital roles in achieving this potential. One disease that can influence future productivity is heifer mastitis caused by Staphylococcus aureus, the coagulase-negative staphylococci (CNS), and the environmental streptococci. The presence of mastitis in young dairy heifers is generally not observed until freshening or until the first clinical flare-up in early lactation. Thus, animals may carry intramammary infections (IMI) for a year or more before they are diagnosed with mastitis, resulting in reduced milk yield (Boddie et al., 1987). The greatest development of milk-producing tissue in the udder occurs during the first pregnancy, so it is important to protect the heifer mammary gland from pathogenic microorganisms to ensure maximum milk production during the first and future lactations.
Prevalence of Mastitis in Unbred and Pregnant Heifers
The initial focus on heifer mastitis began in the mid 1980s after several dairy producers in Louisiana complained to university researchers that a large percentage of their heifers were freshening with clinical mastitis. Subsequent study of breeding-age animals in a research herd revealed that IMI was diagnosed as early as 6 months of age, and that infections persisted throughout pregnancy and into lactation (Boddie et al., 1987). A follow-up study of four commercial herds demonstrated that IMIs were found in 96.9% of heifers and in 74.6% of mammary quarters (Trinidad et al., 1990a). Although the vast majority of udders were visually healthy, 30% of udders and 15.1% of quarters showed clinical symptoms of mastitis as evidenced by clots, flakes and blood; only rarely were quarters swollen and enlarged. Staph. aureus was isolated from 14.7% of quarters. This microorganism was also isolated from 25% of quarters with clinical symptoms. Staph. aureus causes severe damage to mammary tissue, and infections are very difficult to eliminate in lactating cows. Other organisms isolated from secretions and percentage frequencies were: Staph. chromogenes (43.1%), Staph. hyicus (24.3%), other staphylococcal spp. (3.6%), Strep. dysgalactiae (0.4%), Strep. spp. (3.3%), Nocardia species (0.4%) and mixed isolates containing staphylococci and streptococci (5.1%).
 Figure 1. Although the majority of breeding-age dairy heifers are healthy (a), up to 30% show clinical symptoms of mastitis such as ropey, clotted secretions (b); however, enlarged, swollen quarters (c) are rare, thus, this disease is difficult to diagnose via visual observation of the mammary gland.
Figure 1. Although the majority of breeding-age dairy heifers are healthy (a), up to 30% show clinical symptoms of mastitis such as ropey, clotted secretions (b); however, enlarged, swollen quarters (c) are rare, thus, this disease is difficult to diagnose via visual observation of the mammary gland.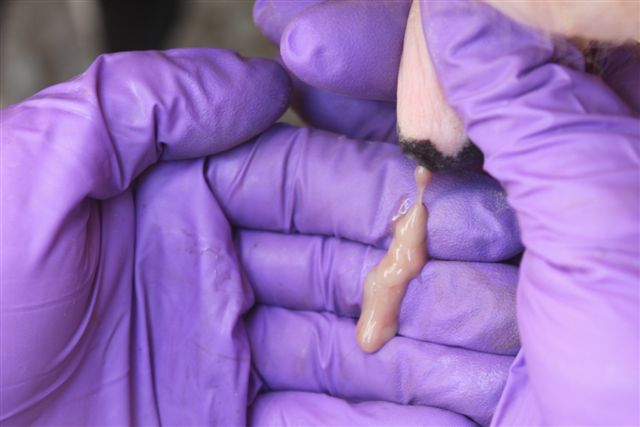 (b)
(b)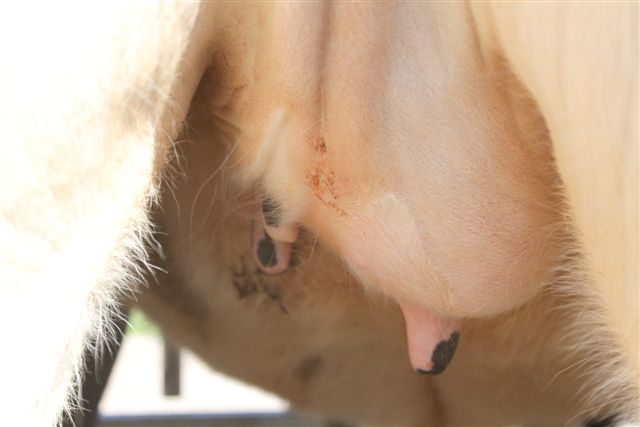 (c)
(c)Mammary Leukocyte Response to Intramammary Infection
In lactating cows, the milk somatic cell count (SCC) is used as a measure of milk quality. This count is composed mainly of leukocytes (macrophages, lymphocytes and neutrophils [Figure 2]) and is considered an important parameter for assessing mammary health status (e.g. inflammation). It is well known that milk yield decreases as the SCC and the incidence of mastitis increases. Thus, SCCs in breeding age and pregnant heifer mammary gland secretions have been analyzed to measure the degree of inflammation and potential reductions in future milk yield. In a study by Boddie et al. (1987), the mean SCC of quarters from unbred heifers infected with Staph. chromogenes, Staph. hyicus and Staph. aureus were 7.8, 8.5 and 9.2 x 106/ml, respectively, whereas the mean SCC of uninfected quarters was 3.5 x 106. Approximately 13% of quarter secretions sampled prepartum contained Staph. aureus, and after freshening, the SCC of these quarters averaged 578 x 103/ml, a cell count associated with a loss of greater than 4.4 pounds of milk per day.
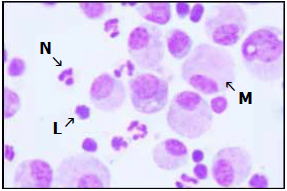 Figure 2. The somatic cell count includes macrophages (M), lymphocytes (L) and neutrophils (N).
Figure 2. The somatic cell count includes macrophages (M), lymphocytes (L) and neutrophils (N).The volume of mammary secretion is very low in breeding-age animals; thus, somatic cells become concentrated, resulting in high SCCs even in uninfected quarters. Such elevated SCCs over a long period of time suggests that mammary tissues in affected quarters are in a state of chronic inflammation, which could adversely affect development of milk-producing tissues and negatively affect future milk yield. In response to infection, neutrophils become the major leukocyte type that infiltrates mammary tissue from the vascular system to phagocytose and kill mastitis-causing bacteria. The migration of neutrophils across the mammary epithelium causes mechanical as well as chemical damage to the milk secretory cells, resulting in decreased yield (Akers and Nickerson, 2011).
Mammary Tissue Response to Presence of Infection
Histologic observations of mammary tissue samples from uninfected quarters of heifers by Trinidad et al. (1990a) showed that milk-producing alveoli were small; the epithelial lining was composed of a single layer of cuboidal cells surrounding a small luminal space with stained secretory product (Figure 3a). Interalveolar connective tissue stroma comprised approximately half of the observed tissue area. Mammary tissues infected with Staph. aureus, on the other hand, exhibited large amounts of interalveolar connective tissue stroma and reductions in alveolar epithelial and luminal areas (Figure 3b), suggesting reduced secretory activity. Indeed, results of morphometric analysis showed that percentages of alveolar epithelium and lumen in quarters infected with Staph. aureus were lower (P < .05) than those in uninfected quarters. Quarters infected with Staph. aureus also showed a greater percentage (P < .05) of interalveolar stroma than did uninfected quarters. Additionally, quarters infected with Staph. aureus exhibited significantly greater infiltration of leukocytes (mainly lymphocytes and neutrophils) compared with uninfected tissues.
The greatest development of secretory tissues in young heifers occurs during the first pregnancy, and these tissues are affected adversely by bacterial infection and inflammation, leading to deposition of connective tissue instead of milk secretory tissue, which results in a reduction of future milk yield.
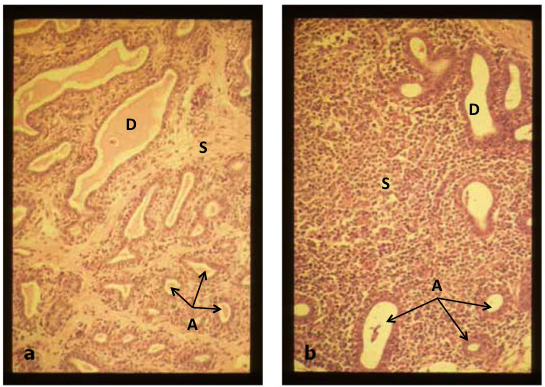 Figure 3a. Portion of mammary parenchymal tissue typical of that obtained from uninfected quarters exhibiting small milk-producing alveoli (A) with ovoid lumens and large ducts (D) containing secretions, and interalveolar connective tissue stroma (S). x180.
Figure 3a. Portion of mammary parenchymal tissue typical of that obtained from uninfected quarters exhibiting small milk-producing alveoli (A) with ovoid lumens and large ducts (D) containing secretions, and interalveolar connective tissue stroma (S). x180.Figure 3b. Parenchymal tissue from a quarter infected with Staph. aureus exhibiting fewer numbers of alveoli (A) and ducts (D) with empty lumens, and a larger percentage of interalveolar connective tissue stroma (S) containing many leukocytes. x180.
Use of Antibiotic Therapy to Control Mammary Infections
Curing existing cases of mastitis with nonlactating cow antibiotic therapy
Because of the high level of infection found in breeding-age and pregnant heifers, especially mastitis caused by Staph. aureus, antimicrobial therapy should be considered. In an initial study to evaluate the effectiveness of treatment, several heifers from four commercial herds were randomly selected to receive a single intramammary treatment of a penicillin and dihydrostreptomycin product into all four mammary quarters (Trinidad et al., 1990b). Treatments were made approximately 60 days prior to calving. Results showed that 97.1% of treated heifers (n = 35) were infected with some type of mastitis at the time of treatment, but, at calving, only 40% remained infected. Of the untreated control heifers (n = 38), 100% were infected at initial sampling, and at calving, mastitis was reduced only slightly to 97.4%. Staph. aureus was isolated from 11 quarters of six treated heifers before antibiotic infusion (45.8%), but at calving this organism was isolated from only one quarter of one heifer (4.2%). In the control group, 18 quarters of 10 heifers were infected with Staph. aureus at time of treatment (45%). At calving, six of the control heifers still had Staph. aureus mastitis in 11 quarters (55%). Thus, the overall incidence of IMI caused by Staph. aureus was reduced more than 90% and SCCs were reduced by 50%. Production data collected over the first two months of lactation demonstrated that Staph. aureus-infected heifers receiving nonlactating cow therapy during pregnancy produced an average of 5.5 pounds more milk per day (or 10% more milk) than Staph. aureus-infected herd mates that did not receive treatment. Other advantages include a longer productive life and higher income due to lower SCC and higher quality milk premiums.
Subsequent studies (Owens et al., 1991, 1994) confirmed results of the initial investigation above. Heifers that were infected with Staph. aureus were infused eight to 12 weeks prepartum with one dose of 300 mg of a cephapirin benzathine product into all four mammary quarters and were compared with untreated controls infected with Staph. aureus. Results demonstrated cure rates between 87% and 100%. After antibiotic infusion, SCC in infected quarters that were cured decreased from 15 x 106/ml to 4 x 106/ml one week later, and to 700 x 103/ml at calving, whereas SCC in untreated controls at calving were 5 x 106/ml. Treated heifers in which Staph. aureus IMI was cured yielded 11% more milk during the first two months of lactation. One of these studies also demonstrated that prepartum treatment served as a prophylactic against new cases of environmental strep mastitis (Owens et al., 1994), reducing the new IMI rate at calving by 93%. Thus, use of nonlactating cow therapy was effective in preventing new IMI as well as curing existing infections.
When is the best time to administer nonlactating cow therapy?
To answer this question, a two-year study was conducted (Owens et al., 2001) in which 233 Jersey heifers were quarter sampled shortly after they were confirmed pregnant and at four-week intervals thereafter. At the initial sampling, 56.5% of quarters were infected with some type of organism, and 15.4% of quarters were infected with Staph. aureus. After the initial sampling, animals were treated with a one-time infusion of one of five different nonlactating cow infusion products during the first (0 - 90 days), second (91 - 180 days) or third (181 - 270 days) trimester of pregnancy. Results showed that cure rates for the five products were high, ranging from 67% to 100%, and significantly higher than the spontaneous cure rate (25%) observed in untreated control quarters. No differences were observed among the three treatment time periods during gestation. Thus, the timing of treatment is best determined by what is most convenient for the management practices of a particular dairy. For example, heifers could be treated: 1) at time of artificial insemination; 2) during routine rectal palpation to determine pregnancy status; or 3) when moved to a new location in preparation for calving. Treatment should be administered no less than 45 days prior to expected calving date to prevent antibiotic residues.
When administering treatment, it is important to 1) restrain the heifer in a headlock and/or squeeze chute, and if necessary further restrain the animal by “tailing”; 2) sanitize the teat orifice with cotton balls or pads soaked in 70% alcohol; 3) use the partial insertion technique when inserting the tip of the syringe cannula (only 2-3 mm) into the teat canal; and 4) dip teats in a germicide to kill any contaminating bacteria. (See Figure 4.)
The treatment of heifers during pregnancy with a nonlactating cow product is advantageous because: 1) the cure rate is higher than during lactation, especially against Staph. aureus; 2) there are no milk losses during therapy; 3) the risk of antibiotic residues is minimal; 4) SCC at calving is reduced; 5) new IMI with environmental streptococci is prevented; and 6) milk production is increased by approximately 10% in successfully treated animals.
 Figure 4. a) Securing the animal in a headlock system while feeding helps to immobilize the heifer. b) Teats should be sanitized prior to treatment. c) When infusing antibiotic, only the tip of the syringe cannula should be inserted into the teat orifice. d) After therapy, teats should be immersed in an effective germicide.
Figure 4. a) Securing the animal in a headlock system while feeding helps to immobilize the heifer. b) Teats should be sanitized prior to treatment. c) When infusing antibiotic, only the tip of the syringe cannula should be inserted into the teat orifice. d) After therapy, teats should be immersed in an effective germicide.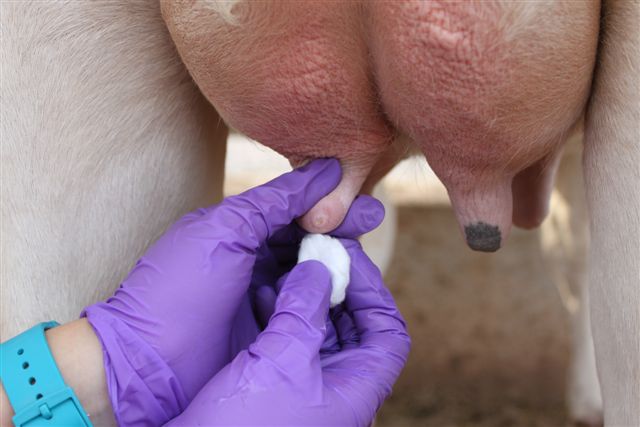 (b)
(b)?
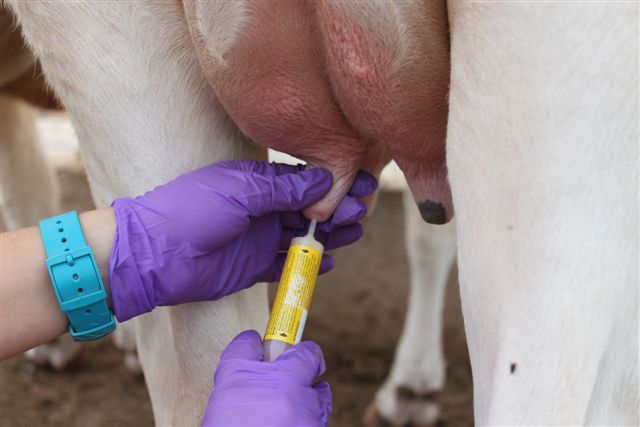 (c)
(c)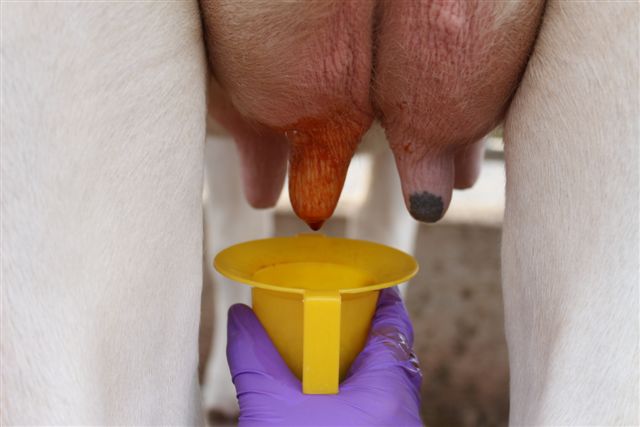 (d)
(d)?
Efficacy of lactating cow products in curing IMI
Lactating cow products also have been used successfully in heifers when treating infections caused by the environmental streptococci and CNS immediately prior to calving. Studies on this subject have been performed in late gestation one to three weeks before calving. Although treating animals one week prior to calving is successful, antibiotic residues often result, so most trials have focused on treating two to three weeks prepartum.
For example, Oliver et al. (2004) conducted a trial to determine if therapy with penicillin-novobiocin or pirlimycin hydrochloride two weeks prepartum was effective in curing IMI and thereby reducing the level of mastitis during early lactation. Approximately 73% of Holstein heifers were infected 14 days before expected calving; the majority of IMIs were due to CNS (44%) and Staph. aureus (30%). At calving, the cure rate was 76% after treatment with penicillin-novobiocin, and 59% following treatment with pirlimycin. In this same study, 96% of Jersey heifers were infected 14 days before calving; the majority of IMIs were due to CNS (61%), Strep. spp. (19%) and Staph. aureus (8%). At calving, the cure rate was 75% after treatment with penicillin-novobiocin, and 87% following treatment with pirlimycin. Thus, prepartum therapy of heifer mammary glands with penicillin-novobiocin or pirlimycin hydrochloride was effective in reducing the percentage of heifers infected with mastitis pathogens during early lactation.
As a part of the above trial, milk production and SCC data from 82 control heifers and 111 heifers treated with antibiotics before calving were evaluated (Oliver et al., 2003). Milk production was about 10% higher in heifers treated prepartum with antibiotics. Additionally, treated heifers had significantly lower SCCs than control heifers (~52,000 vs. 81,000). Thus, prepartum antibiotic treatment to reduce the rate of mastitis in heifers during early lactation was economically beneficial.
The studies on prepartum treatment with lactating cow therapy administered seven to 21 days before calving have shown treatment to be effective for quarters infected with CNS and Strep. uberis, but waiting until this time to treat chronic Staph. aureus mastitis might be too late. A mammary gland that has been infected with Staph. aureus for several months to a year will not develop normally, and treatment during the immediate prepartum period would most likely be of little benefit in curing infections or salvaging mammary tissue. At this point, the tissue damage would have already been done, and affected quarters should have been treated earlier in gestation to: 1) cure existing infections; 2) reduce chronic inflammation; and 3) allow mammary tissue to develop normally during the later stages of pregnancy.
Results of these trials demonstrated that nonlactating and lactating cow antimicrobial treatment of heifers known to be at risk for developing IMI is advantageous because the cure rate is much higher than that realized when treating infections during lactation. In addition, most studies show that SCCs are lower, there is no milk loss due to therapy, risk of antibiotic residue at calving is minimal and future milk production is increased in heifers cured of IMI. However, the goal in controlling mastitis is to prevent new infections, and management strategies to enhance disease prevention are discussed below.
Managing Heifers to Prevent Mammary Infections
Role of vaccination in mastitis control
Although antimicrobial therapy is successful in curing existing cases of mastitis, the goal from a herd management perspective is to prevent new infections from occurring, and vaccination has been attempted as a prophylactic measure. The purpose of vaccination is to increase circulating antibodies directed against certain mastitis pathogens to protect against bacterial invasion. Researchers in Louisiana evaluated a commercially available Staph. aureus vaccine in young dairy animals (Nickerson et al., 1999). This product is the only Staph. aureus vaccine on the market in the U.S. (Lysigin?, Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO, USA). At 6 months of age, 35 Jersey heifers were vaccinated following manufacturer?s instructions using a 5 ml dose intramuscularly in the semimembranosus muscle (upper thigh) of the rear leg (Figure 5), and 14 days later, animals received a booster dose, which was repeated at six-month intervals. Another 35 heifers served as unvaccinated controls. (Note: Injections were administered per label instructions; however, it is generally recommended to avoid muscle tissues in the leg, thereby minimizing potential abscess formation; injection into the neck region is preferable.) Results demonstrated that: 1) the number of quarters exhibiting chronic Staph. aureus mastitis during pregnancy was reduced 43.1% in vaccinates compared with controls; 2) the rate of new IMIs during pregnancy was reduced 44.8%; 3) the rate of new IMIs at freshening was reduced 44.7%; and 4) the SCC was reduced by 50% in vaccinates compared with controls.
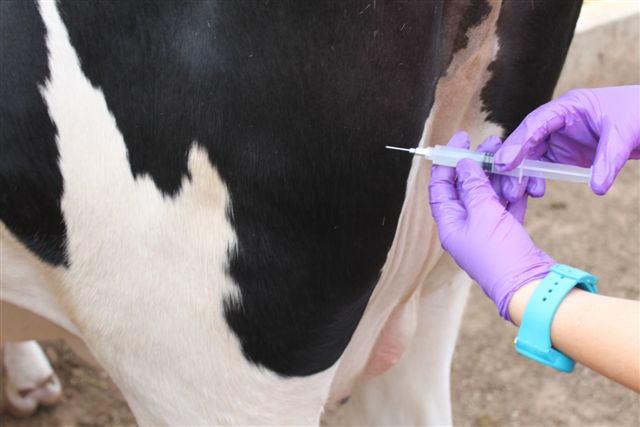 Figure 5. Vaccination (5 cc) into the left semimembranosus muscle of the rear leg using an 18-gauge needle. (Note: Injections were administered per label instructions; however, it is generally recommended to avoid muscle tissues in the leg, thereby minimizing potential abscess formation; injection into the neck region is preferable.)
Figure 5. Vaccination (5 cc) into the left semimembranosus muscle of the rear leg using an 18-gauge needle. (Note: Injections were administered per label instructions; however, it is generally recommended to avoid muscle tissues in the leg, thereby minimizing potential abscess formation; injection into the neck region is preferable.)In a subsequent, more in-depth study using the same vaccine (Lysigin?), 106 Holstein heifers from a dairy herd in Virginia were evaluated (Nickerson et al., 2009). Previous microbiological culture of heifer mammary secretions indicated that approximately 35% of the animals were infected with Staph. aureus. At 6 to 18 months of age, 53 heifers were vaccinated and boosted as above, and the other 53 heifers served as unvaccinated controls. The purpose was to determine if vaccination reduced the level of Staph. aureus at calving as observed in the Louisiana trial.
Vaccine efficacy data showed that the percentage of heifers with Staph. aureus IMI at freshening was lower in vaccinates (13.3%) compared with controls (34.0%) -- a reduction of 60.9%. Likewise, an examination of health records showed that the percentage of heifers that were culled or died during the trial was reduced by approximately one-third by vaccination: 16.9% in vaccinates and 24.5% in controls. Somatic cell counts in samples collected during first week of lactation from uninfected heifers for vaccinates and controls were 66,095 and 132,754/ ml, respectively (a 50.2% reduction), and for infected heifers, SCCs were 441,764 and 892,176/ml, respectively (a 50.5% reduction).
An examination of the 305-day lactation milk yield for the first lactation of both vaccinated and unvaccinated control heifers demonstrated an approximate 10% increase in production in vaccinates vs. controls (24,250 vs. 22,046 pounds, respectively) or a difference of 2,204 pounds. Likewise, the 305-day pounds of both fat and protein were higher in vaccinates than controls (fat: 899 vs. 747 kg, respectively; protein: 727 vs. 694 kg, respectively). An examination of the number of days in milk for the first lactation demonstrated that vaccinates lactated 13 days longer than the unvaccinated controls (349 vs. 336 days). In addition, average first lactation SCCs were 11,000 cells/ml lower in vaccinates compared with controls (49,000 vs. 60,000/ml).
Results of this Virginia investigation demonstrated that vaccinating dairy heifers according to the prescribed protocol with a commercial USDA-licensed Staph. aureus bacterin, Lysigin?, reduced the number of new Staph. aureus intramammary infections at time of calving by 60.9%, lowered the SCC by 50% and decreased the culling rate by approximately one-third. In addition, overall milk yield, production of fat and protein, and number of days in milk were greater in vaccinated heifers compared with controls. This prevention strategy may represent a major control mechanism for managing Staph. aureus in the future, especially as new antigens and adjuvants are added to vaccine preparations.
Efficacy of an infusible teat seal for preventing IMI
Because bacteria breach the teat canal to cause infection, products have been developed to serve as a barrier to bacterial entry or seal the teat canal against infection. Such products form a physical plug in the distal teat cistern and teat canal (Figure 6). In one study involving 255 pregnant heifers, mammary quarters were treated approximately one month prepartum with an infusible teat seal composed of bismuth subnitrate. Results showed that this procedure: 1) reduced the risk of new IMI with any organism by 74%, 2) reduced the prevalence of postcalving IMI by 65%, 3) reduced the risk of Strep. uberis infection in quarters with an IMI pre-calving by 70%, and 4) reduced the incidence of clinical mastitis from which a pathogen was isolated by 70% in quarters detected with an IMI pre-calving (Parker et al., 2007). In a subsequent trial, 1,067 bred heifers were treated approximately one month prepartum with teat seal, and results demonstrated that treatment: 1) reduced the risk of clinical mastitis by 68% and 2) reduced the risk of IMI due to Strep. uberis by 84% (Parker et al., 2008).
Although teat seal contains no antibiotic and cannot cure existing infections, this product is effective in preventing new cases of mastitis, especially those caused by Strep. uberis. It is emphasized that to be effective, teat seal should be administered approximately 30 days prior to calving and that strict teat end hygiene be followed when applying teat seal products.
The role of horn flies in the development of heifer mastitis
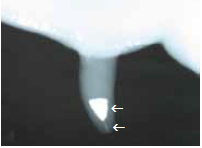 Figure 6. Radiograph of a teat infused with teat seal, illustrating the teat seal material in the distal teat cistern and in the teat canal (arrows).
Figure 6. Radiograph of a teat infused with teat seal, illustrating the teat seal material in the distal teat cistern and in the teat canal (arrows).Historically, the major association between flies and intramammary infections has been with the development of summer mastitis, in which the biting fly, Hydrotoea irritans, is the proven vector. Summer mastitis is an isolated seasonal problem primarily in July, August and September in heifers and dry cows of northern Europe, and may be controlled by insecticidal sprays. In the U.S., fly control is used to reduce these insect pests on farm premises, and subsequently reduce animal stress, but its application as an adjunct management practice for preventing new cases of mastitis and reducing SCC has not been considered or embraced by producers.
An initial survey performed at Louisiana State University showed that prevalence of mastitis in bred heifers was significantly lower in dairy herds that used some form of fly control for their lactating cows, dry cows and heifers compared with herds applying no fly control (Figure 7) (Nickerson et al., 1995). The greatest reductions were in numbers of Staph. aureus and the environmental streptococci, both major mastitis pathogens in adult cows associated with elevations in SCC.
?
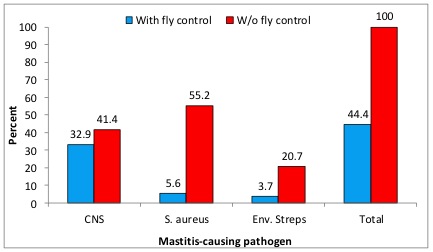 Figure 7. Prevalence of mastitis in Louisiana dairy herds with and without a fly control program.
Figure 7. Prevalence of mastitis in Louisiana dairy herds with and without a fly control program.The particular species of fly associated with mastitis is the blood-sucking horn fly (Haematobia irritans). This species is commonly found on the backs of dairy animals (Figure 8a), preferring a dark hair coat, but will also attack the teats, leading to the development of mastitis, especially among dairy heifers. Results of the survey above (Nickerson et al., 1995) also demonstrated that bred heifers having teats with bite lesions and scabs caused by horn flies exhibited a 70% frequency of IMI compared with a 40% frequency in heifers with normal teats free of lesions. Such infections are always associated with elevated SCC in excess of 5 million/ml in these young animals. Horn flies tend to attack front teats rather than rear teats. See Figure 8b below illustrating horn flies and lesions on heifer teats.
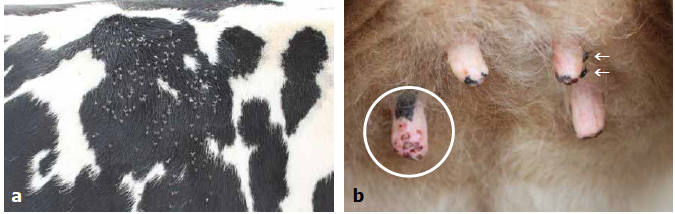 Figure 8. a) Horn flies on the back of a Holstein heifer; note the preference for dark hair color vs. white hair. b) Udder of a 10-month-old heifer illustrating horn flies (arrows) and lesions on teat ends.
Figure 8. a) Horn flies on the back of a Holstein heifer; note the preference for dark hair color vs. white hair. b) Udder of a 10-month-old heifer illustrating horn flies (arrows) and lesions on teat ends.?
Since that first survey, researchers have proven through DNA studies that the horn fly is not only responsible for teat lesions on heifers, but is indeed a vector in the transmission of mastitis-causing bacteria such as Staph. aureus, from heifer to heifer, especially during the warm and humid months of the year (Owens at al., 1998). Such mastitic heifers serve as sources of IMI for transmission to the entire lactating and nonlactating herds.
How can flies be controlled to manage heifer mastitis?
Once it was established that the horn fly was a vector in the transmission of mastitis-causing bacteria, the next step was to develop management practices to reduce flies and lower the prevalence of intramammary infections. Insecticide-impregnated tags placed on the tail switch in close proximity to the udder during the spring and summer months were successful in reducing horn fly populations by 60% as well as the incidence of mastitis during the first two months after placement. However, after two months, tags fell off and replacing them was impractical from a management standpoint (Nickerson et al, 1997). In a subsequent trial, the daily dietary supplementation of an insect growth regulator helped to suppress fly populations but not enough to prevent new cases of mastitis in dairy heifers (Owens et al., 2000). However, the use of an insecticidal pour-on every two weeks for six weeks followed by treatment with insecticidal ear tags reduced fly populations and decreased the incidence of new Staph. aureus IMIs by 83% during a six-month trial in heifers during the warm season in Louisiana (Owens et al., 2002). More recently, an ongoing trial at the University of Georgia has found that the use of a pour-on every two to four weeks drastically reduced fly populations, allowing teats to heal, and reducing two important sources of S. aureus: flies and teat lesions.
These studies demonstrate that during the warm and humid months of the year, horn flies do serve as vectors in the transmission of heifer mastitis, which is associated with elevated SCC in these young dairy animals. Although research has not been conducted to show this same association in lactating and dry adult cows, it is assumed that fly populations play some role in the elevation of mastitis and SCC observed in the hot summer months. And, with the proposed reduction in the SCC legal limit to 400,000/ml in the USA, and in light of the fact that milk buyers are imposing their own limits, some as low as 250,000/ml, it is imperative that dairymen utilize all possible means to prevent new cases of mastitis and their associated SCC. A simple fly control program can serve as an important adjunct to an overall herd plan of mastitis control and assist dairymen in lowering their bulk tank SCC and earning quality premiums for their products.
Influence of dietary supplementation on mammary health
Another management tool to reduce the level of infection and SCC when heifers calve, as well as throughout lactation, is through dietary supplementation. Diet plays a role in udder resistance to infection because certain nutrients affect various mammary resistance mechanisms, namely: (1) leukocyte function, (2) antibody transport and (3) mammary tissue integrity. In one study, heifers received selenium (0.3 ppm/day) and vitamin E (50 to 100 ppm/day) supplementation starting 60 days prepartum and throughout lactation, and a selenium booster injection (50 mg) was administered 21 days prior to freshening (Hogan et al., 1993). This protocol reduced staphylococcal and coliform infections at calving by 42%, and duration of infection was reduced 40% to 50% in supplemented heifers. Clinical mastitis in supplemented heifers was reduced 57% in early lactation and 3.2% throughout lactation, and the mean SCC was lower. Thus, vitamin E and selenium improved udder health of heifers, and the effect of dietary supplementation was most evident at calving and in early lactation.
In a more recent study (Eubanks et al., 2012), dairy heifers (n = 40) were fed a daily supplement beginning at 5 months of age containing an immunostimulant (OmniGen-AF?), which included a proprietary mixture of B-complex vitamins and yeast extract. Blood profile data collected during gestation showed that compared with unsupplemented control animals (n = 40), those supplemented with the immunostimulant exhibited greater leukocyte expression of L-selectin and interleukin-8 cell surface receptors, as well as greater leukocyte phagocytic activity and production of reactive oxygen species, suggesting the capability for a greater immune response to bacterial infection. Shortly after freshening, results from 20 heifers that calved (Note: study in progress) showed that supplemented animals exhibited lower SCC than controls (180 vs. 711 x 103/mL) and lower prevalence of mastitis (4% vs. 13%). At 14 days postpartum, supplemented animals produced a mean daily yield of 55.1 pounds per day compared to controls producing 48.1 pounds per day; a 7-pound increase. Although the trial is still ongoing, results thus far demonstrate that dietary supplementation with OmniGen-AF improved udder health and milk quality postpartum, resulting in greater milk yield in early lactation. From a practical standpoint, feeding of such immunostimulants would be a suitable and effective tool for dairy producers to utilize for controlling mastitis in their herds.
Summary
Prevalence of mastitis in unbred, breeding-age and pregnant dairy heifers is higher than formerly realized. Infected mammary quarters, especially those with Staph. aureus IMI, exhibit reduced mammary gland secretory potential, marked leukocyte infiltration and the accompanying inflammation. Both nonlactating and lactating commercial antibiotic infusion products have been used successfully to cure existing infections and reduce SCC, and nonlactating therapy prevents new IMI with environmental streptococci. However, the goal is to prevent new infections from occurring in these young dairy animals through management strategies aimed at vaccination, use of teat seals, fly control and dietary supplementation. Results of commercial vaccine trials illustrate that immunization will reduce Staph. aureus mastitis in heifers at calving between approximately 45% and 60%, with reductions in SCC of 50%. Likewise, a fly control program for heifers will decrease incidence of Staph. aureus mastitis by up to 83%. Lastly, dietary supplementation to boost the immune systems of heifers has been shown to reduce incidence of mastitis at calving, lower SCC and increase milk yield. As global milk quality standards become more stringent, management practices based on curing existing infections and preventing new IMI in heifers will ensure that these young dairy animals enter the milking herd free of mastitis and with low SCC. Such practices should be considered for incorporation into dairy herd health programs in herds suffering from a high prevalence of heifer mastitis, especially mastitis caused by Staph. aureus. Not only do these practices reduce new infections in first-calf heifers at parturition, they also reduce the introduction of Staph. aureus to the milking herd.
References
Akers, R.M. and S.C. Nickerson. 2011. Invited review. Mastitis and its impact on structure and function in the ruminant mammary gland. Journal of Mammary Gland Biology and Neoplasia. 16:275-289.
Boddie, R.L., S.C. Nickerson, W.E. Owens and J.L. Watts. 1987. Udder microflora in nonlactating heifers. Agri- Practice, Vol.8, pp. 22-25.
Eubanks V.J., D.J. Hurley, L.O. Ely, F.M. Kautz, S.C. Nickerson, N.E. Forsberg, Y.Q. Wang, K. Zanzalari and J. Chapman. 2012. Pre- and postpartum immunomodulatory effects of a dietary supplement on the immune system of dairy heifers. J. Anim. Sci. Vol. 90, Suppl. 3/J. Dairy Sci. Vol. 95, Suppl. 2. Abstr #220, pg. 222.
Hogan, J.S., W.P. Weiss and K.L. Smith. 1993. Role of vitamin E and selenium in the host defense responses to mastitis. Journal of Dairy Science, Vol.76, pp. 2795-2802.
Nickerson, S.C., W.E. Owens and R.L. Boddie. 1995. Mastitis in dairy heifers: Initial studies on prevalence and control. Journal of Dairy Science, Vol.78, pp. 1607-1618.
Nickerson, S.C., W.E. Owens, S.M. DeRouen, R.L. Boddie and G.M. Tomita. 1997. Use of insecticide impregnated tail tags may reduce incidence of mastitis in beef cows. The Louisiana Cattleman. August, Pages 11-14.
Nickerson, S.C., W.E. Owens, G.M. Tomita and P.W. Widel. 1999. Vaccinating dairy heifers with a Staphylococcus aureus bacterin reduces mastitis at calving. Large Animal Practice, Vol.20, pp. 16-20.
Nickerson, S.C., L.O. Ely, E.P. Hovingh and P.W. Widel. 2009. Immunizing dairy heifers can reduce prevalence of Staphylococcus aureus and reduce herd somatic cell counts. In: Dairy Cattle Mastitis and Milking Management, DAIReXNET.
Oliver, S.P., M.J. Lewis, B.E. Gillespie, H.H. Dowlen, E.C. Janicke and R.K. Roberts. 2003. Milk production, milk quality and economic benefit associated with prepartum antibiotic treatment of heifers. Journal of Dairy Science, Vol.86, pp. 1187-1193.
Oliver, S.P., S.J. Ivey, B.E. Gillespie, M.J. Lewis, D.L Johnson, K.C Lamar, H. Moorehead, H.H. Dowlen, S.T. Chester and J.W. Hallberg. 2004. Influence of prepartum intramammary infusion of pirlimycin hydrochloride or penicillin-novobiocin on mastitis in heifers during early lactation. Journal of Dairy Science, Vol.87, pp. 1727-1731.
Owens, W.E., S.C. Nickerson and R.L. Boddie. 2000. The effect of methoprene on horn fly numbers and mastitis in dairy heifers. Large Animal Practice, Vol.21, pp. 26-28.
Owens, W.E., S.C. Nickerson, R.L. Boddie, G.M. Tomita and C.H. Ray. 2001. Prevalence of mastitis in dairy heifers and effectiveness of antibiotic therapy. Journal of Dairy Science, Vol.84, pp. 814-817.
Owens, W.E., S.C. Nickerson, P.J. Washburn and C.H. Ray. 1991. Efficacy of a cephapirin dry cow product for treatment of experimentally induced Staphylococcus aureus mastitis in heifers. Journal of Dairy Science, Vol.74, pp. 3376-3382.
Owens, W.E., S.C. Nickerson, P.J. Washburn and C.H. Ray. 1994. Prepartum antibiotic therapy with a cephapirin dry cow product against naturally occurring intramammary infections in heifers. Veterinary Medicine Series B, Vol.41, pp. 90-100.
Owens, W.E., S.P. Oliver, B.E. Gillespie, C.H. Ray and S.C. Nickerson. 1998. Role of horn flies (Haematobia irritans) in Staphylococcus aureus-induced mastitis in dairy heifers. American Journal of Veterinary Research, Vol.59, pp.1122-1124.
Owens, W.E., S.C. Nickerson and C.H. Ray. 2002. Effect of a pour-on and fly tag insecticide combination in controlling horn flies and Staphylococcus aureus mastitis in dairy heifers. 2002 Louisiana Dairy Report. Baton Rouge, Louisiana, USA, pp. 39-42.
Parker, K.I., C. Compton, F.M. Anniss, A. Weir, C. Heuer and S. McDougall. 2007. Subclinical and clinical mastitis in heifers following the use of a teat sealant precalving. J. Dairy Science, 90: 207-218.
Parker, K.I., C. Compton, F.M. Anniss, C. Heuer and S. McDougall. 2008. Quarter level analysis of subclinical and clinical mastitis in primiparous heifers following the use of a teat sealant or an injectable antibiotic, or both, precalving. J. Dairy Science, 91: 169-181.
Trinidad, P., S.C. Nickerson and R.W. Adkinson. 1990a. Histopathology of staphylococcal mastitis in unbred dairy heifers. Journal of Dairy Science, Vol.73, pp. 639-647.
Trinidad, P., S.C. Nickerson, T.K. Alley and R.W. Adkinson. 1990b. Efficacy of intramammary treatment in unbred and primigravid dairy heifers. Journal of the American Veterinary Medical Association, Vol.197, pp. 465-470.
?
Status and Revision History
Published on Feb 28, 2013
