In the summer of 2010, bermudagrass (Cynodon dactylon (L.) Pers.) hay producers in Georgia counties (JeffDavis, Irwin, Pierce, and Tift) began noticing a “bronzing” of their hay fields, generating damage similar to that of severe drought- or frost-damaged bermudagrass (Figure 1A). The bronzing was the result of chlorosis and necrosis in the top two to three leaves of the plant (Figure 1B). The damaged leaves could easily be pulled fromthe sheath, and the end inside the sheath either showed evidence of insect damage or obvious decay (Figure 1C).The collected larvae were grown out and allowed to pupate and mature. The resulting adults were subsequently identified as Atherigona reversura Villeneuve (Diptera: Muscidae), now commonly known as the bermudagrass stem maggot (BSM).



The BSM is believed to be native to Southeast Asia, which is where it was first discovered. Since the 2010 discovery in southern Georgia, the BSM has spread throughout the Southeast, damaging bermudagrass turfgrass, hayfields, and pastures as far north as North Carolina and Kentucky and as far west as Texas.
Yield Losses
In general, each Atherigona species has its own preferred host. Though it may be found on or around other grass species, A. reversura has only been found to damage bermudagrass and stargrass (C. nlemfuensis) in the United States.
The larva of BSM bores into the pseudostem, the stem-like structure made up of leaf sheaths, where it macerates the vascular tissue. It feeds on the sap and microbial soup that it creates from the macerated tissue. This feeding occurs outward from the last node of the plant, which cuts off water and sap flow to and from the top two to three leaves. The amount of yield loss caused by this feeding depends upon the stage of growth wherein the damage occurs.
If the damage occurs once the bermudagrass is nearing harvest, the loss of those top two to three leaves may reduce the yield by less than 10% for that cutting. However, if the damage occurs during the early stages of regrowth, affecting less than 6 inches (15 centimeters) of new growth, yield losses can be severe. Yield losses in excess of 80% have been reported in bermudagrass hayfields in the latter part of the season.
Since bermudagrass is grown for hay and pasture—around 300,000 and 3 million acres, respectively, in Georgia alone—on so many acres across the Southeast, the economic impact of the BSM is substantial. Conservatively, a total yield loss of up to 3 tons/acre (6,700 kg/ha) could be expected in a typical bermudagrass hayfield in south Georgia if the BSM was left untreated. Depending on the quality and market for this forage, a loss of 3 tons/acre could represent an economic loss of over $600/acre ($1,500/ha). Preliminary research has shown that BSM damage decreased relative feed quality (RFQ) of late-season bermudagrass hay by 7% on average. This decrease was attributed to lower total digestible nutrients (TDN) and slightly lower dry matter intake (DMI). Crude protein (CP) actually increased in the damaged bermudagrass, but this was a function of dilution of desirable carbohydrates. This would be similar to the phenomenon seen in weathered hay such that CP is actually higher in the outer edges of the hay bale where desirable carbohydrates have leached out of the bale while the nitrogen remains.
Growth Stages
Like other species in the Muscidae family, the adult stage of the BSM is a fly. The BSM fly is easier to find and identify than the larva or pupae because it occurs outside of the pseudostem and has distinct coloration (Figure 2). They have transparent wings, a gray thorax, and a yellow abdomen with at least one pair of black spots. Adult BSMs are about 1/8 inch (around 3.0 to 3.5 millimeters) in length. The females are typically larger than the males. The female abdomen is longer, more pointed, and curves under the fly’s body. In contrast, the male’s abdomen is shorter and more rounded. The proportion of female to males in a population varies from field to field and, perhaps, with the season. Data collected to date indicate that the females outnumber the males by an average of 4.6 to 1. However, this ratio has been observed to vary from 2:1 to 10:1.
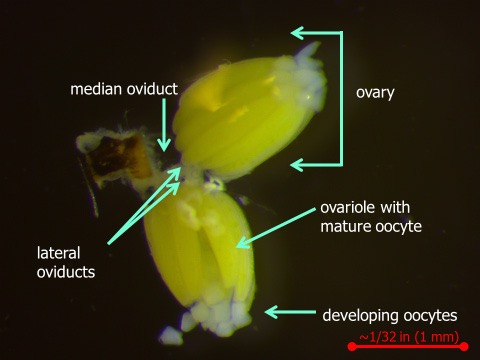
The BSM female has the potential to lay a large number of eggs. Figure 3 shows the two ovaries of the female’s reproductive tract. Within each ovary, there are approximately 15 to 18 ovarioles. Each ovariole is capable of producing an oocyte (egg).
The larvae are white, cylindrical, and about 1/8 inch (3 millimeters) long when fully grown (Figure 4A). As they mature, their color gradually darkens to a tan or brown. The larvae also have mouth hooks that are barely visible to the naked eye. It is presumed that these mouth hooks enable the BSM to macerate the walls of the pseudostem. The metamorphosis of the BSM larvae into the adult fly occurs in a puparium, a rigid outer shell covering the pupae, that is orange to dark red and barrel-shaped, similar to that of other Atherigona species.


Life Cycle
A better understanding of the bermudagrass stem maggot’s life cycle and biology has emerged as research on the timing of these biological phases continues. The life cycle begins with the BSM fly laying an egg on a bermudagrass leaf. The larva emerges approximately two to three days post-oviposition, or after eggs are laid, and slips or bores into the central whorl of the pseudostem. Once in the pseudostem, it begins to macerate the vascular tissue at the first node it encounters.
The lack of sap flow causes the top two to three leaves to become chlorotic, or yellowed due to insufficient clorophyll. Within one to two days after feeding begins, the first signs of damage are observed, and the affected leaves soon become completely chlorotic or necrotic (prematurely dying). Between the time when chlorosis is first observed and complete leaf senescence, or deterioration, the larva exits the stem (Figure 4B) and moves to the soil for pupation. The metamorphosis occurs in an orange-colored puparium over the course of 7 to 10 days and culminates with the emergence of the adult fly.
It has also been shown that when the pseudostem is cut (with a hay mower or grazed, for example), any viable larvae will exit the stem and move to the soil to pupate. As a result, adult flies begin to emerge in a sizeable flush 7 to 10 days after cutting. These findings have helped refine the timing for insecticide applications for suppressing adult populations during the first two to three weeks following a cutting (see the “Chemical Control” paragraph in the “Mitigation Strategies” section).
Adult flies live for approximately 18 to 20 days when kept in enclosures and provided sugar water. Actual adult life spans are estimated to be 14 to 21 days. Based on these observations, we believe the complete life cycle of the BSM to be three to four weeks long with multiple offspring being produced by the fly during its adulthood.
The degree to which the BSM overwinters in the Southeast remains unclear. We have observed that populations increase progressively from south to north, with high populations developing as early as mid-June in central Florida, early July in south Georgia, mid-July in central Georgia, and late July in north Georgia and points further north. This would indicate that overwintering success is, at a minimum, much better in more southern climes. Nonetheless, we have collected flies as early as February near Valdosta, Georgia, and mid-May near Athens, Georgia, so we presume they have at least some ability to overwinter at these latitudes.
Assessing BSM Populations
The orange, barrel-shaped puparium of the BSM may be found just under the soil surface when the insect is pupating. However, finding puparia in the soil has proven too challenging for a practical assessment of BSM populations. Consequently, no protocols have been developed to search for the pupae in a damaged field’s soil.
Finding the larva is only slightly less challenging, as it requires dissecting pseudostems as soon as they show the first signs of chlorosis in response to BSM damage. If the pseudostem shows extensive damage, then it is likely the larva has already left the pseudostem to pupate. Pseudostems may be carefully dissected using a sharp knife or razor blade, splitting the stem until the center of the shoot is revealed. Because of the small size of the larva, it is best to work over a solid, dark-colored surface so that the larva is not lost during the procedure.
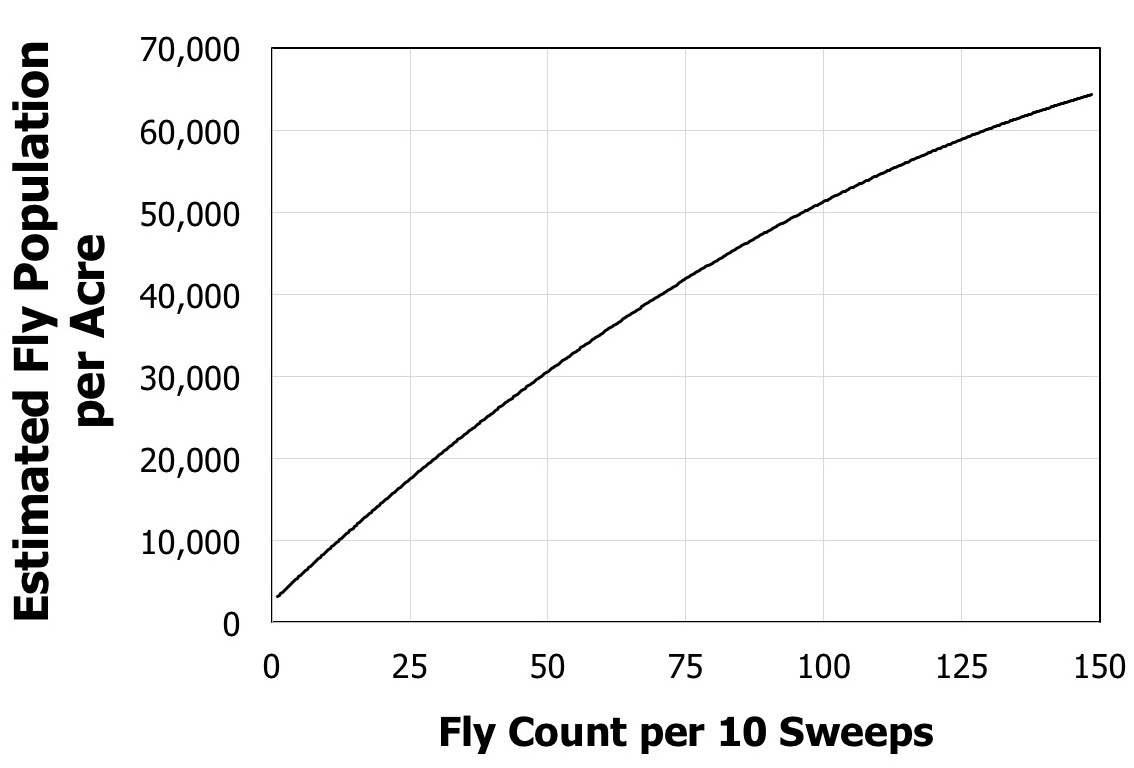
Producers can use sticky traps or sweep nets to collect and identify the BSM fly in the field relatively easily (Figure 5). However, they tend to stay down in the forage canopy and rarely fly higher than 18 inches (0.5 meter) above the canopy. To date, sticky traps have only been useful in alerting one to the presence of the BSM because fly counts on sticky trap cards have not yet been observed to be correlated with fly populations. Sweep net estimates have been found to be relatively accurate predictors of actual fly populations in the field (Figure 6). Correlating fly populations to actual yield loss has proven much more challenging than simply catching and counting flies. The amount of damage is not merely a function of fly populations because a number of other factors also can influence yield loss. These include, but are not limited to, bermudagrass variety, timing of the infestation, number and proximity of bermudagrass fields near the field in question, timing of when those neighboring fields were last cut, amount of disease present in the crop, and the amount of fertilization added to the crop.




Varietal Differences
Research into damage by the BSM has shown that varieties that produce finer leaves or pseudostems and/or produce more pseudostems per square foot (or square meter) tend to be more susceptible to damage and yield loss. In general, the coarse-textured varieties of stargrass (Cynodon nlemfuensis Vanderyst) and hybrids of bermudagrass and stargrass (cv. ‘Tifton 85’, ‘Coastcross-I’, and ‘Coastcross-II’) are less susceptible to BSM damage. While these varieties have fewer tillers, proportionately fewer of those tillers are damaged. Cultivars with a higher number of shoots also tend to have a smaller shoot diameter, narrower leaves, and a lighter green color. These plant characteristics create a denser forage canopy, which seems to attract the BSM (Figure 7).
Consequently, some varieties of bermudagrass are more susceptible to damage by the BSM than are others. Table 1 reports the common yield loss observed in research trials conducted by the University of Georgia and the U.S. Department of Agriculture’s Agricultural Research Service (USDA-ARS) in Tifton, Georgia, as well as observations made by 禁漫天堂 Extension agents and bermudagrass producers in Georgia. Though substantial yield loss can certainly be possible in the less susceptible varieties, performance is generally greater. Ongoing plant breeding efforts at the USDA-ARS in Tifton have shown progress in developing more varieties that are high yielding and high quality while exhibiting more tolerance to the BSM. Until then, producers should choose the most tolerant variety that is recommended for their area.
|
Variety |
Typical Range in Yield Loss Observations (%) |
|
Sprigged |
|
|
Alicia |
30-60* |
|
Coastal |
15-30* |
|
Coastcross II |
0-15* |
|
Russell |
20-40* |
|
Tifton 44 |
15-30 |
|
Tifton 85 |
0-20* |
|
Seeded |
|
|
Common |
30-60* |
|
Various seeded |
30-60 |
Table 1. Varieties of bermudagrass differ in the amount of yield loss typical observed in harvests made after the second cutting.
Range observed in yield trials comparing treated and untreated plots.

Timing of Infestation
When BSM damage occurs near the end of a regrowth cycle (within 2.5 to 3 weeks after the previous cutting or grazing), the yield loss is usually less than 10%. However, a bermudagrass crop damaged at an early stage of regrowth (e.g., 6 to 8 inches or 15 to 20 centimeters) is unlikely to further develop. Bermudagrass is very intolerant of shade, especially in regards to producing new tillers or pseudostems. When the top of a 6-to-8- inch-tall crop (15 to 20 centimeters) is damaged and left in the field, it will cast enough shade to slow or stop new pseudostem emergence. Thus, the crop growth slows or ceases. If the crop is damaged at this point, it is crucial to remove the damaged grass to enable new growth to occur.
Infestation timing is also related to the number and proximity of bermudagrass fields near the field in question and the timing of when those neighboring fields were last cut. Experience has shown that bermudagrass fields surrounded by forest or row crops tend to be less susceptible to damage. Fields of bermudagrass that are large or near large fields are most susceptible. Whenever these neighboring fields are harvested, the flies in those fields will often move into fields with bermudagrass that can sustain the population. This buildup of BSM population on a field that is just a few days into a regrowth cycle can result in heavy damage and yield losses. Consequently, producers should be aware of the harvest schedule of neighboring fields and take action to control the BSM when damage is likely.
Growth Conditions Influence Damage
Increased BSM damage is frequently associated with fields suffering from heavy disease pressure or bermudagrass stands receiving high rates of nitrogen (N) and low rates of potassium (K) fertilizer. It is still unclear why these conditions are associated with increased BSM damage. A balanced soil fertility program minimizes the risk of disease and ensures a healthy stand.
Mitigation Strategies
As with any pest, one should employ an integrated pest management strategy that exploits biological, cultural, physical, and/or chemical control measures. Although Atherigona populations are unlikely to be fully controlled (much less eradicated), taking an approach that integrates these control measures will reduce economic damage.
Biological Control – Since it is a non-native species, none of the BSM’s natural predators are present in the Southeast, to our knowledge. It is presumed, however, that some insect and spider species present in bermudagrass pastures and hayfields would prey on the BSM. However, the extent and significance of this predation on controlling the BSM population is unknown. Thus, the most successful tool for biological control is to choose a variety that is tolerant or less susceptible to BSM damage. As discussed in the section entitled “Varietal Differences,” bermudagrass producers should choose varieties that are the least susceptible among the bermudagrass varieties recommended for their area.
Cultural Control – Bermudagrass stands that are managed to minimize disease pressure and fertilized with a balance of nutrients are generally less susceptible to BSM damage. Interseeding alfalfa into bermudagrass has substantially reduced or eliminated BSM damage in bermudagrass hayfields. This practice, which has benefits beyond eliminating BSM damage (e.g., reducing or eliminating N fertilization needs, lowering fertilizer costs, increasing forage quality), should be strongly considered whenever a bermudagrass stand proves to be prone to damage.
Physical Control – Properly timed bermudagrass harvests can minimize the yield losses from the BSM. If signs of BSM damage occur near the end of a regrowth cycle (within 2.5 to 3 weeks after cutting or grazing), the producer should harvest or graze the field as soon as conditions become favorable. Once a stand that is 6 to 8 inches (15 to 20 centimeters) or taller has been damaged by BSM feeding, the only option is to cut and/or graze the stand to a height of 3 to 4 inches (7.5 to 10 centimeters) and encourage regrowth to occur. It is better to cut the field extremely early and accept the loss than to have a low-yielding, severely damaged crop that harbors a large fly population and leads to a further buildup.
Ideally, the infected material would be removed from the field to prevent shading of any regrowth. The larvae do not appear to remain in cut stems. Within hours of cutting, larvae will exit damaged stems and travel to the soil. Flies in fields that have been harvested escape to field margins and neighboring bermudagrass fields. Prompt applications of chemical controls in fields following the harvest of a neighboring field can greatly reduce the risk of BSM damage.
Chemical Control – Chemical control of the BSM larva is challenging because it is inside the pseudostem. Consequently, an insecticide with systemic activity would be needed to prevent larval feeding. However, none of the systemic insecticides currently approved for use in pastures or hay crops are labeled for (or effective at) controlling the BSM. Consult your county Extension agent for specific pesticide recommendations.
The BSM fly is the target of chemical suppression efforts. A broad spectrum insecticide timed when large numbers of adult flies are present provides the most suppression. Suppression of the BSM fly can be challenging because the flies are mobile. In our experience, the flies do not fly very high (usually less than 18 inches, or 0.5 meters, above the canopy) nor very far (no more than 10 feet, or 3 m) in any single instance of flight, even after being disturbed. Therefore, normal spray boom heights should be effective for chemical applications for BSM control. However, it is also important to understand the limits of a chemical application in canopy penetration. In our experience, the BSM flies tend to remain deep in the canopy except to move from one location to another or in response to a disturbance. Applications that do not penetrate the canopy may have limited success. It would be ideal to apply the insecticide in a volume of water in excess of 12 to 15 gallons/acre (112 to 140 L/ha) to ensure adequate canopy penetration.
Suppressing the BSM can be effective when a recommended rate of an insecticide is applied after the bermudagrass has begun to regrow (7 to 10 days after cutting) following an affected harvest. A second application can be made 7 to 10 days later to suppress any flies that have emerged or arrived since the last application. This second application is usually only necessary when neighboring fields were harvested after the first application, the crop growth cycle has been extended due to dry weather, or a forecast of rain suggests that the hay harvest may be delayed. Chemical actions should be taken if there is a known history of BSM damage to the bermudagrass and the expense of the application(s) is justified by the forage yield saved. An individual application usually costs $2 to $3/acre ($5 to $7.50/ha) for the insecticide and $5 to $10/acre ($12 to $25/ha) for application. If the bermudagrass forage is valued at $100/ton ($90/metric ton), a corresponding yield savings
of approximately 200 lbs DM/acre (225 kg DM/ha) would be necessary to warrant this investment. In July and August, bermudagrass hayfields may produce up to 6,000 lbs DM/acre (6,725 kg DM/ha) at a single cutting, so applications at this time of year are more likely to result in an economic benefit. Because bermudagrass yields in September or October may only be 1,500 to 2,000 lbs DM/acre (1,700 to 2,250 kg DM/ha), fall insecticide applications are much less likely to result in a return on that investment.
Based on our current observations, BSM populations are not high enough to warrant chemical suppression prior to the first bermudagrass hay cutting (or equivalent timing if the crop is to be grazed) and population buildup may not occur until late into the regrowth cycle for the second cutting for the central latitudes of the Southeast U.S. or the third cutting for more northern areas where bermudagrass is grown.
Insecticide Resistance – Overuse of pesticides of a single mode of action to combat a pest that is capable of producing a large number of offspring is likely to eventually result in a buildup of resistance to that pesticide’s mode of action. Care should be taken to avoid using insecticides too frequently and extensively and occasionally changing the mode of action used.
Much remains unanswered about the BSM. Additional research is needed to identify economic thresholds and alternative pesticides that differ in their mode of action. The current information provides producers basic management and suppression techniques, but much more research is needed to assist producers in making informed decisions about options for BSM management.
References
Baxter, L. L. (2014). Bermudagrass stem maggot: An exotic pest in the Southeastern USA [Master’s thesis]. University of Georgia.
Baxter, L. L., Hancock, D. W., & Hudson, W. G. (2014). The bermudagrass stem maggot (Atherigona reversura Villeneuve): A review of current knowledge. Forage and Grazinglands, 12(1), 1–8.
Baxter, L. L., Hancock, D. W., Hudson, W. H., Dillard, S. L., Anderson, W. F., & Schwartz, B. M. (2015). Response of selected bermudagrass cultivars to bermudagrass stem maggot damage. Crop Science, 55(6), 2682–2689.
McCullers, J. T. (2014). Sampling techniques and population estimation for Atherigona reversura Villenueve (Diptera: Muscidae) in bermudagrass hay fields [Master’s thesis]. University of Georgia.
Acknowledgements

Much of the work conducted on the BSM has been funded by grants from the Georgia Beef Commission, formally known as the Agricultural Commodity Commission for Beef. The authors wish to thank the GBC’s board and the beef and dairy producers of Georgia.
Status and Revision History
Published on Nov 15, 2017
Published with Full Review on Jun 09, 2023
