Sweet potatoes are members of the Convolvulaceae family, which includes morning glory. They are native to tropical Central and South America and are a perennial plant there. In more temperate regions, such as the United States, they are frost sensitive and grown as annuals. Sweet potatoes are grown for their edible root, which is often mistakenly called a tuber, like white or Irish potatoes (Solanum tuberosum). However, the edible portion of the sweet potato is a true root and will continue to enlarge as long as the plant continues to grow (Rubatzky and Yamaguchi, 1997). Sweet potatoes have been grown by Native Americans in Mexico, Central America, Peru, Ecuador, and the U.S. for thousands of years.
In the past, Georgia was a leading sweet potato producer for the U.S. But the emergence of the weevil Cylas formicarius (Fabricius) caused Georgia’s sweet potato production to decline significantly. Over the past several years, however, acreage in Georgia has rapidly increased, with growers capitalizing on market demand and successfully using sweet potato as a rotation for winter-grown vegetable crops. Currently, there are about 5,000 acres of sweet potato grown commercially in Georgia. North Carolina leads the country in production with more than 80,000 acres grown commercially.
Cultivating Sweet Potato
Cutting production
Sweet potatoes are vegetatively propagated with sprouts (slips or cuttings) taken from seed beds, which are then planted for the production of the commercial crop. Sweet potato roots do not experience dormancy but tend to produce new shoots at the “stem” end of the root (Figure 1). Growers may produce their own or purchase cuttings or certified seed stock.

Production of sweet potato plants begins with tissue culture plantlets called “Generation 0.” After plants are cultured, they are typically grown out in a greenhouse setting. Cuttings from these plants (now Generation 1) are then planted to produce what’s known as “foundation seed.” Foundation seed roots are stored and used as a source of Generation 2 plants the following year. Generation 2 plants can be sold as cuttings or grown for Generation 2 certified seed, which can be purchased the following year as certified seed stock. Growers can then grow their own cuttings using Generation 2 certified seed or by saving seed from their previous crops, which is often considered Generation 3 or 4. It is highly recommended that a portion of a grower’s seed stock be replenished with Generation 2 certified seed annually to maintain vigor and quality in the seed stock.
To produce cuttings, it is recommended that seed stock be planted in the field after all chance of frost is diminished. Seed stock can be kept at temperatures of 75 °F and 90% relative humidity for a period of two weeks prior to planting in order to “presprout” and encourage rapid growth once planted. Seed roots can then be placed end-to-end in plant beds in the field and covered with 2 to 3 inches of soil (Figure 2A). Seed beds are usually covered in a clear plastic mulch to increase soil temperatures and encourage sprouting (Figure). It is advisable to punch holes in the mulch; otherwise, seed beds can become anaerobic, resulting in poor sprouting and root rots (Figure 2C). Soil temperatures in the range of 82 to 86 °F are ideal for sprout production. After shoots begin emerging from the soil, producers should split open the clear plastic and pull it to the sides of the bed to prevent new shoots from being burned by hot plastic (Figure 2D). Cuttings can be taken from seed beds when they are approximately 10 to 12 inches long and are usually taken 1 to 2 inches above the soil line to prevent transfer of soilborne diseases like scurf. Multiple cuttings may be taken from the same seed beds, usually two to three weeks after the first cutting. A complete granular or water-soluble fertilizer should be applied to seed beds to encourage rapid growth and regrowth after cutting. Traditionally, sweet potato “slips” were planted instead of cuttings. A sweet potato slip is essentially a shoot that has been pulled from the seed potato and includes roots, with some portion of the slip having grown below the soil line. Slips are rarely used today due to the enhanced likelihood of spreading soilborne diseases and the ability to take multiple cuttings from plant beds.




Producing cuttings requires a significant amount of seed stock. Depending on the plant spacing and the productivity of the seed stock, roughly 500 to 650 lb of seed stock are required to produce 1 acre of cuttings (Coolong et al., 2012). Cuttings should not be allowed to dry out prior to planting and can be stored at 60 to 65 °F. Do not store cuttings in a refrigerated cooler below 60 °F, as low temperatures can inhibit rooting and potentially cause injury to cuttings.
Variety selection
The ‘Covington’ cultivar currently claims more than 80% of the acreage planted in Georgia, with additional acreage devoted to ‘Beauregard’ and other varieties like ‘Evangeline.’ All of these varieties have typical orange skin and deep orange flesh. ‘Murasaki’ is a purple-skinned variety with a white flesh that is grown for some specialty markets, and ‘O’Henry’ is a brown- or tan-skinned variety with white flesh. Ongoing variety trials are being conducted to identify any new varieties that may be of interest to Georgia growers.
Planting
Sweet potatoes can be successfully planted once soil temperatures are above 65 °F and the danger of frost has passed. However, the most limiting factor for planting time is the availability of cuttings for planting. Most cuttings grown in Georgia or North Carolina are not available until late May. Therefore, growers who want to plant earlier may need to arrange to buy cuttings from regions of south Florida or grow them in a protected structure such as a high tunnel.
A range of plant populations are used to grow sweet potatoes in Georgia. However, most growers use rows spaced 36 to 42 inches on center, based on their existing equipment spacing. Within-row spacing typically ranges from 10 to 14 inches (Table 1). Slightly closer in-row spacing (10 inches) may result in increased competition and, in our trials, has resulted in a small reduction in jumbo-sized roots at a given harvest time.
Sweet potato beds are often formed prior to planting in order to apply preplant incorporated insecticides for soilborne pest control. Sweet potatoes are generally planted using a finger-style transplanter for bare-root plants reminiscent of those used for tobacco production. Carousel-type planters are not as suitable, as the cuttings do not have enough weight to fall through the carousel and the leaves of the cuttings may get caught in the planting cup. Water is often applied at transplanting, and a mild starter solution (10-34-0 N-P-K) or similar is often applied at a rate of 0.5 to 1 pint per 50 gallons of transplant water.
Table 1. Sweet potato plant populations per acre at different row (in column) and within-row spacing (in rows).
|
Row Spacing (inches) |
Within Row Spacing (inches) |
||
|
10 |
12 |
14 |
|
|
30 |
20,908 |
17,424 |
14,984 |
|
36 |
17,420 |
14,520 |
12,450 |
|
40 |
15,840 |
13,200 |
11,310 |
|
42 |
14,940 |
12,450 |
10,670 |
|
44 |
14,280 |
11,900 |
9,330 |
Fertility requirements
Sweet potatoes perform poorly in the heavy clay soils of north Georgia, so fertility recommendations are based on the sandier soils found in the Coastal Plain region of the state. In general, it is recommended that 90-110 lb of nitrogen per acre be applied for the production of sweet potatoes in the Coastal Plain of Georgia. Typically, a small portion of nitrogen, 25-30%, is applied preplant during bed formation, with the remainder applied over two to three applications during the season. The majority of nitrogen can be applied by the time vines completely cover row-middles. Generally it is recommended to apply all necessary phosphorous either preplant or shortly after planting. While sweet potatoes are generally light nitrogen feeders compared with other vegetable crops, they require high levels of potassium fertilization (Table 2). Fertilizer recommendations are provided in Table 2. Sweet potatoes grow over a wide range of soil pH values and successful crops can be obtained between a pH of 5.5 to 6.5. Lower pH values (<5.2) can reduce the incidence of scurf (Rubatzky and Yamaguchi, 1997).
Table 2. Crop removal rates and current fertilizer recommendations for sweet potatoes grown in the Coastal Plain of Georgia.
|
Nutrient |
Removal Rates (lb/acre) |
|||||
|
Roots |
Shoots |
Total |
||||
|
Nitrogen |
39 |
46 |
85 |
|||
|
Phosphorous |
17 |
7 |
24 |
|||
|
Potassium |
160 |
90 |
250 |
|||
|
Fertilizer Needed (lb/acre) Coastal Plain |
||||||
|
Nitrogen |
90-110 |
|||||
|
Phosphorous |
Low P |
Medium P |
High P |
Very High P |
||
|
180 |
120 |
60 |
30 |
|||
|
Potassium |
Low P |
Medium P |
High P |
Very High P |
||
|
180 |
120 |
60 |
30 |
|||
Nutrient removal rates adapted from Scott and Bouwkamp (1974) and Rubatzky and Yamaguchi (1997).
Growth
Although it may not look as if much is happening during early-season growth, the time period during the first 28 days after planting is critical to development, as this is when the number of storage roots per plant is determined. After 28 days of transplanting, the number of storage roots changes little and from this point until harvest is when they “bulk up” (Gajanayake et al., 2014). Soil temperatures can have a significant impact on root numbers as well. The number of storage roots initiated per plant begins to decline with high soil temperatures. Studies
in Mississippi showed a significant increase in root biomass with air and soil temperatures of approximately 80 °F (Gajanayake et al., 2014). By late June, average soil temperatures in southern Georgia routinely exceed 82 to 84 °F during the day, suggesting that root initiation may be reduced in late-planted sweet potatoes. High temperatures also negatively impact root growth more than shoot growth, suggesting that late-planted sweet potatoes may appear to have good vine growth while lacking adequate root growth.
Irrigation
Although sweet potatoes may be perceived as drought tolerant compared to other vegetables, irrigation throughout the entire life cycle of the crop is important. Optimal soil moisture regimes were found to be between 72 and 100% of evapotranspiration. Recent findings from on-farm demonstrations in southeast Georgia have found that 1-inch weekly irrigations, while sufficient for early-season growth, are not adequate to maintain soil moisture levels later in the season when vine growth is extensive. Research is ongoing to determine ideal irrigation levels in Georgia, but preliminary work suggests that 1.5 inches per week split into 2 or 3 irrigation events is preferred. Interestingly, drought stress may not appear obvious in vine growth, but has dramatic effects on root growth.

Figure 3 shows roots from the variety Covington planted within 2 days of each other, but one in a non-irrigated field (right) and the other in a field receiving 1-inch per week of irrigation (left). Neither field showed any obvious differences in aboveground growth, but clearly differed in growth rate. Growers should also avoid allowing fields to dry only to irrigate infrequently but heavily. Large fluctuations in soil moisture can result in root splitting (Figure 4).

Harvest
Harvest times for sweet potatoes are dependent on growing degree-days and range from 76 days to more than 110 days for the same variety grown in southeast Georgia. Generally, it is recommended to begin periodically test-digging short (50- to 100-foot) sections of rows late in the season. This is the best way to determine whether your crop is ready to harvest. Based on the data obtained from growers in southern Georgia, sweet potatoes are usually harvested when 65-75% of harvested tubers belong to U.S. grade No. 1. Sweet potatoes will continue to grow and one may risk having too many jumbo roots if harvest is delayed for too long.
It is recommended to mow sweet potatoes several days prior to digging to help harden the skin and make it easier for harvest crews to walk through fields. However, if heavy rains are expected, it is recommended not to mow in order to reduce the chance of disease. Chemical desiccants are not recommended, as they may cause an increase in ethylene production, which can lead to poor storage life (J. Schultheis, personal communication).
Most sweet potatoes in Georgia are dug using a flip plow, which is less likely than a chain digger to cause damage to the fragile skin of a root. Digging with a flip plow requires significant labor as sweet potatoes are manually picked after they are flipped out of the ground. Do not allow sweet potatoes to sit exposed to the sun for more than 1-1.5 hours after harvest, as they can easily become sunburned. It is also noteworthy that the labor required to harvest sweet potatoes is much greater than the labor required to plant. While a grower may easily plant 50-60 acres per day, they may only be able to harvest one quarter of that same acreage in a day. Producers may need to plan the harvest schedule when planting so as not to have sweet potatoes growing too large in the field.
Postharvest
After harvest, sweet potatoes need to be cured at temperatures of 80 to 85 °F and 85-90% relative humidity for approximately one week. Curing is essential to strengthen the fragile skin on sweet potatoes and to allow any wounds to heal. It also enhances the flavor of the crop by increasing the simple sugars in the roots. After curing, roots are stored at 55 to 60 °F at 85-90% relative humidity until they are boxed for shipping. Generally, roots are cured prior to cleaning, as running freshly dug roots on the packing line prior to curing could cause significant damage. An excellent resource for postharvest management of sweet potatoes can be found at: https://content.ces.ncsu.edu/postharvest-handling-of-sweetpotatoes.
Sweet potatoes are typically sold in 44-lb boxes. Grading standards are often determined by shippers or buyers, but USDA size standards are as follows.
-
U.S. No. 1 should be between 1.75-3.5 inches in diameter, 3-9 inches long, and no more than 20 ounces.
-
U.S. No. 1 Petite should be between 1.5- 2.25 inches in diameter and 3-7 inches long.
Sweet potatoes are also expected to be clean, firm, well-shaped, free of decay and insect damage or other mechanical damage. Complete USDA grading guidelines can be found at https://www.ams.usda.gov/grades- standards/sweet potatoes-grades-and-standards.
Managing Diseases of Sweet Potato
Viral diseases
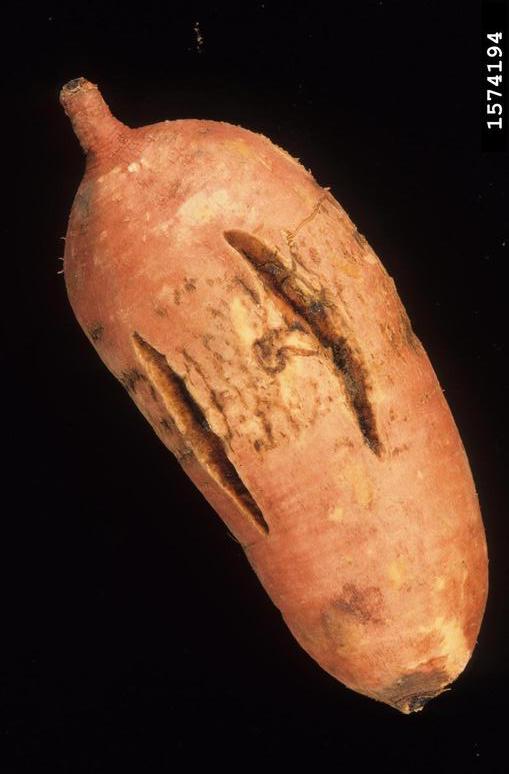
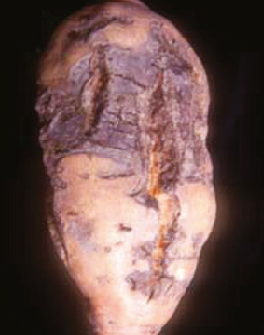
Sweet potato feathery mottle virus (SPFMV): Feathery mottle is caused by a potyvirus called “sweet potato feathery mottle virus,” or “SPFMV.” Symptoms can be seen on foliage as irregular, chlorotic spots with occasional purplish pigment at the border. Chlorosis (feathering) can also be observed along midribs. Symptoms on storage roots include radial and longitudinal surface cracks. SPFMV strain (russet strain) can cause external necrotic lesions and occasional internal corking (Figure 5).
SPFMV is transmitted by a wide range of aphid species in a nonpersistent manner. The virus can also be introduced on infected slips/cuttings. Visual screening of slips/cuttings for SPFMV is difficult as the infected material may remain asymptomatic.
Management: Being a nonpersistent virus transmitted by aphids, vector control may not be feasible. Use of resistant varieties remains the most important management strategy against this virus.
Sweet potato sunken vein virus (SPSVV): This virus belongs to the closterovirus group and is transmitted by whiteflies (Bemisia tabaci). Symptoms on plants may include vein yellowing, sunken spots on secondary veins on the upper surface of the leaf, and swollen veins on the lower surface of the leaf. In extreme cases, SPSVV may cause stunting, but this is rare.
The virus is transmitted by whiteflies in a semipersistent manner. It can also be introduced on infected slips/ cuttings like SPFMV. Generally, SPSVV and SPFMV occur as a mixed infection, causing severe symptoms. An infection of SPSVV alone may cause mild symptoms, but a mixed infection with SPFMV more often leads to SPVD (see below), which can be more damaging.
Management: The use of resistant varieties is key for the management of this disease.
Sweet potato virus disease (SPVD): This disease often occurs as a result of a mixed infection of SPFMV and SPSVV, but it is unknown if other viruses are also involved as a virus complex. The symptoms of SPVD include severely stunted plants with small and narrow (strap-like) leaves. Other foliar symptoms like puckering, vein-clearing, and mottling may also occur. In severe cases, total yield losses have been experienced.
Management: Vector control may not be feasible. This disease can be managed with the use of resistant varieties.
Bacterial diseases
Bacterial stem and root rot: Dickeya dadantii (previously known as Erwinia chrysanthemi)
This disease is caused by a gram-negative bacterium (Dickeya dadantii). The pathogen survives in the soil on plant debris and weeds and can be spread through contaminated irrigation water. However, infection often requires wounding on stems or tubers.

The bacterium can cause both aboveground and belowground symptoms. Aboveground symptoms are observed on stems and petioles (leafstalks) as water- soaked, brown to black lesions (Figure 6a). In some cases, wilting can be seen on one to two branches, and as the disease progresses, entire plants may die.
Belowground symptoms include lesions on fibrous and fleshy roots. On tubers, lesions with dark margins can be seen with no obvious rotting symptoms. However, when tubers are cut through these lesions, internal rot can be observed (Figure 6b).

Management: Using resistant cultivars and avoiding tissue damage and wounding can reduce disease incidence.
Soil rot: Streptomyces ipomoea
This disease is caused by a soilborne bacterial pathogen, Streptomyces ipomoea. This disease is problematic in dry, alkaline soils. Aboveground symptoms include extensive chlorosis and bronzing of leaves. These symptoms can be related to damaged fibrous roots due to extensive infection. This bacterium can also damage the storage roots, which can develop dark-brown necrotic lesions associated with cracks on the surface. Dumbbell-shaped malformations can also occur on the surface (Figure 7).

Management: Use planting material from areas where this disease has not been reported. Keeping the crop in optimum soil moisture conditions may help in reducing disease incidence. Use of sulfur can reduce soil pH and ultimately disease incidence. A five-year rotation with a nonhost crop is recommended.
Fungal diseases
Black rot: Ceratocystis fimbriata
This fungal disease is characterized by dark sunken spots or cankers in
the lower part of the stem. Severe infection can also lead to wilting and death. On storage tubers, dark, sunken spots with fungal signs (protruding, black, spine-like structures) can be seen (Figure 8). The pathogen is spread by infected planting stock (slips). Pathogen transmission can also occur through wounds caused by sweet potato weevils, wireworms, crickets, and mice. The pathogen is soilborne and remains in plant debris for at least one to two years.

Management: Avoid getting cuttings/slips from infected plants. Cuttings should be made 0.78 inch above the soil line. Two- to three-year rotations with nonhost crops are recommended. It’s beneficial to treat seed roots with fungicide prior to planting.
Circular spot: Sclerotium rolfsii
Circular spot of sweet potato is caused by the soilborne basidiomycete fungus, Sclerotium rolfsii. This pathogen has a wide host range, including peanut, tomato, pepper, and other agronomic crops. Symptoms include circular, light- to dark-brown sunken spots with defined margins. When cross-sections are taken through the sunken spots, lesions may appear to be saucer-shaped (Figure 9). As the disease progresses, lesions can become dry, hard, and leathery and in some cases can be removed. The tissue below the lesion can be bitter to taste. This is a field disease and infected lesions do not spread after harvest as with other postharvest pathogens.


Management: Avoid planting in fields with a history of southern blight.
Scurf: Monilochaetes infuscans
This disease is caused by a soilborne fungal pathogen, Monilochaetes infuscans. The pathogen survives in soil or plating beds. Initial symptoms can be seen
as dark brown spots on the surface of the tubers, which gradually enlarge and coalesce (Figure 10). The lesions are superficial on the skin. Aboveground parts are generally not affected. Healthy roots can be contaminated upon contact with contaminated carets, baskets and storage houses.

Figure 10. Scurf (Monilochaetes infuscans) causes grayish brown to black lesions on the skin of the root.
Management:
-
Use healthy planting material.
Use certified seed to grow disease-free plants. While planting carefully inspect roots for symptoms. Do not plant infected roots. Wetting the roots makes scurf spots easier to see. Treat seed with labeled fungicide before planting; please refer to the Georgia Pest Management Handbook. Vine cuttings or cut sprouts should be used for planting. These planting materials can be cut above soil level and should be dipped in fungicide prior to planting; please refer to the Georgia Pest Management Handbook. Disinfest contaminated equipment before using. -
Find a planting location that’s free of scurf.
Locate fields/sites with no history of scurf or with at least three years free of sweet potato. -
Crop rotation for three to four years is recommended.
Rhizopus soft rot: Rhizopus nigricans
Rhizopus soft rot is caused by Rhizopus nigricans. This disease can occur at both field and postharvest. The tubers can get infected during harvest through injuries on the surface. The injured tissues provide avenues for pathogen ingress and infection. The infected tissue becomes soft and water-soaked
and eventually rots (Figure 11). Fungal growth (whiskers) can be seen on the infected tissue (Figure 11). Rotten tissue emits unpleasant fermented odor, which further attracts fruit flies.

Management: Ensure minimum to no damage or wounding to tubers before, during and after harvest. Avoid bruising or rough handling of tubers during transport and storage. Cure tubers after harvest to assist in periderm formation on wounds. Other management strategies are similar as those described for scurf.
Diseases caused by nematodes
Root-knot nematodes
Root-knot nematodes are important pests of sweet potato causing considerable reduction in the quality and yield of storage roots. Infested growing areas might contain more than one species of root-knot nematodes. The southern root-knot nematode, Meloidogyne incognita, represents the most commonly distributed species in sweet potato in the Southern U.S. In recent years, however, a new devastating species, M. enterolobii (guava root-knot nematode), has been confirmed infesting sweet potato in Louisiana, North Carolina, and South Carolina. The guava root-knot nematode is capable of breaking resistance in certain crops including sweet potato. Other species include M. javanica, M. hapla and M. arenaria (McSorley, 1980; Johnson et al., 1996), but these species are not as damaging to sweet potato as M. enterolobii or M. incognita.
Biology and symptoms: Root-knot nematodes cause severe changes in
the physiology and morphology of the host plants. Infected plants have reduced growth and vigor and they may wilt permanently. The infective second-stage juveniles of the nematodes enter the roots of the sweet potato plant, reproduce and produce eggs rapidly, and cause little to severe galling on the roots. Root-knot nematodes can complete a generation time in approximately three to four weeks in suitable environmental conditions. The most obvious symptoms of Meloidogyne infection in sweet potato are deformed and cracked tubers as well as knotted roots (Figure 12) (Clark and Moyer 1988; Jatala, 1991; Overstreet, 2009). The nematodes produce some small to large bumps and dark lesions on the surface of tubers (storage roots). The lesions contain nematode feeding sites that provide places for invasion of other pathogenic and/or secondary microorganisms, which may intensify infection by creating a disease complex and/or microbial degradation of storage roots. There is a competitive interaction between the reniform nematode and M. incognita, and both species are capable of increasing cracks on sweet potatoes (Thomas and Clark, 1983). Root-knot nematodes are more problematic in sandy soils compared with other soil types. Relatively high temperature and moisture conditions may lead to an increase in root-knot reproduction and development, which may subsequently cause changes in the incidence and dispersal of the nematode. Such changes may have a detrimental impact on sweet potato production, particularly if susceptible varieties are grown.

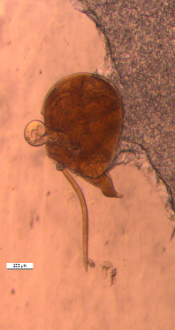
Control: Successful sweet potato production requires that growers use several strategies to reduce losses to root-knot nematodes. Applying chemical fumigants and non-fumigant nematicides alone or in combination with cultural methods, such as rotation with nonhost crops, are among the most effective methods of managing root-knot nematodes. Treating soil with fumigants prior to planting may be the only reliable and effective option for control of root-knot nematodes. Resistant sweet potato varieties will reduce production costs and farmers’ reliance on nematicides and will also increase marketable yields. Commercial varieties of sweet potato, such as ‘Covington’ (Yencho et al., 2008) and ‘Evangeline’ (La Bonte et al., 2008), have resistance to M. incognita and are available in the U.S. These varieties, however, are susceptible to M. enterolobii. No source of resistance to M. enterolobii in sweet potato is currently available. Meloidogyne enterolobii has a very wide host range including cotton, tobacco, corn, wheat, soybean, many vegetables, fruits, and ornamentals that make the nematode control extremely difficult.
Reniform nematode
Rotylenchulus reniformis is a polyphagous nematode infesting about 300 plant species. Sweet potato is an excellent host to reniform nematode. In the U.S., M. incognita was previously reported as the most challenging parasite of sweet potato, but recently R. reniformis has risen in importance to growers. In the Southern U.S., the reniform nematode occurs mainly in regions of intense cotton production (Heald and Robinson, 1990; Koenning et al., 2004; Overstreet, 2009) with high population densities occurring in light, sandy soils.
Biology and symptoms: The reniform nematode generally completes its life cycle in host plants between 24 to 29 days at optimum environmental conditions. A potential risk for production of sweet potato in soils with high reniform nematode populations is that the damage may go undetected. Primary symptoms caused by R. reniformis are root necrosis and reduced root systems. Overall stunting and yellowing of foliage, as well as cracks and distortions on roots, can be observed on infected plants, which reduce the quality of the sweet potato. Nematode damage can also be detected if female nematodes are visible on the root surface. The nematode is capable of causing considerable yield loss if it’s not managed (Clark and Wright, 1983; Abel et al., 2007).
Control: The most common method of controlling R. reniformis and optimizing sweet potato yield is the use of fumigants or nematicides before planting. Additionally, several botanical nematicides are available that can be used as post-plant treatments to manage infestation from R. reniformis. Rotating sweet potato with nonhost crops between growing seasons has shown potential for managing the reniform nematode. Marigolds (Tagetes spp.) and sunn hemp (Crotolaria juncea) can be used as as cover crops that can suppress R. reniformis populations in infested fields (Wang et al., 2001). Rotating sweet potato with some resistant varieties of cotton or soybean followed by a fallow period may also help to reduce the number of nematodes in the soil (Jatala, 1991; Abel et al., 2007).
Lesion nematodes
Lesion nematodes, Pratylenchus spp., are known to parasitize sweet potato in some countries. The three common species associated with sweet potato are P. brachyurus, P. coffeae, and P. flakkensis. In the U.S., P. brachyurus is reported from sweet potato-growing regions, but little is known about its economic importance (Overstreet, 2009).
Biology and symptoms: Both P. brachyurus and P. coffeae cause small to extended necrotic lesions on feeder roots as well as storage roots of sweet potato. Pratylenchus brachyurus causes a reduction in crop yield and marketability. Additionally, secondary fungal or bacterial microorganisms can enter these lesions causing root rot (Jatala, 1991; Scurrah et al., 2005).
Control: Sweet potato varieties with resistance to P. coffeae and P. flakkensis are available in Japan and Peru, which have reduced producer dependence on nematicides. Preplant soil treatment with fumigants and granular nematicides can effectively reduce the nematode populations below the damage threshold.
Stem and tuber rot nematodes
Ditylenchus dipsaci and D. destructor are important pathogens of sweet potato that cause significant losses wherever they occur. In China, both nematode species limit the quality and productivity of sweet potato (Zhang et al., 2006).
Biology and symptoms: Juveniles and adults of Ditylenchus species attack sweet potato roots. Except some stem necrosis and poor growth, little or no distinctive symptom can be detected on the aerial parts of the plant. In contrast, the nematodes produce brown to brownish-black layers on tubers that often become decayed following invasion by other secondary microorganisms. Yield can be negatively affected when high numbers of D. dipsaci and D. destructor are present in soil (Scurrah et al., 2005; Overstreet, 2009).
Control: Because these nematodes favor high temperatures (71.6 to 75.2 °F) for growth and reproduction, storing roots at cooler conditions may reduce damage. Resistance to D. dipasci and D. destructor has been found in some varieties of sweet potato (Jatala, 1991; Scurrah et al., 2005).
Other nematodes of sweet potato
Several other genera of plant-parasitic nematodes have been reported on sweet potatoes, including burrowing nematode, Radopholus similis, spiral nematode, Helicotylenchus dihystera, sting nematode, Belonolaimus longicaudatus, and stubby root nematodes, Paratrichodorus minor and Trichodorus spp. These nematodes, however, are not of major economic importance on sweet potato. Soil fumigation and the use of nematicides can effectively reduce population densities of these nematodes below the damage threshold.
Managing Insect Pests of Sweet Potato
Numerous insects can feed and reproduce on sweet potatoes, but relatively few are consistent economic pests. The pests and insect stages of greatest concern are those that feed directly on the harvestable portion of the crop, the storage roots. These pests occur in the soil and are the larval stages of various beetles including white grubs, wireworms, rootworms, and the sweet potato weevil. The sweet potato weevil is unique among these pests in that all stages can occur in the roots and it can complete its lifecycle in roots in storage, making it the most severe storage pest. A variety of insects will feed on the foliage of sweet potatoes, but these are generally of minor concern as sweet potatoes can tolerate a great deal of defoliation without yield impact. The sweet potato whitefly has shown potential to impact growth and development in some years and may be becoming a more consistent pest of sweet potato. The presence of defoliators at harvest time may present a threat as they may feed on roots when the foliage is removed and the roots are exposed for harvest.
Soil insect pests
Wireworms
Wireworms bore shallow holes into the surface of sweet potato roots. Damage early in the season will heal but may also appear deeper as the roots expand. Multiple species of wireworm attack sweet potato (Figure 13). Wireworms are the larval stages of click beetles (Figure 15). Wireworms are cylindrical, cream to yellowish-orange with reddish-brown heads and a reddish brown “tail,” and they are smooth and relatively hard (thus the common name “wireworm”). Final instar wireworms are generally 1/2 to 3/4 of an inch long. The adults are small, flat, reddish-brown to dark-brown beetles that cause no damage to sweet potato (Figure 14). The presence of wireworms in the soil can be monitored with bait stations (a mixture of corn and wheat is most effective) placed in the soil 3 to 4 inches deep and removed 10 to 14 days later. The life cycle of wireworms varies by species and may be three to four months with multiple generations per year or may require one or more years for a single generation (Figure 13). Wireworms overwinter in the soil and may be present in the field prior to planting or enter the field during the production season. Eggs are laid in the soil near potential host plants.




White grubs
White grubs bore large shallow holes into sweet potato roots. Grubs are relatively large (up to 1 inch), dirty white, C-shaped larvae with brown head capsules and three pairs of true legs near the head (no prolegs) (Figure 16). White grubs are the immature stages of May and June beetles. These pests overwinter in the soil and a single generation requires one
to three years depending on species. Adults are active primarily in May and June and lay eggs in the soil with a preference for grasses, pastures, or weedy fields. White grubs can be present in the soil prior to planting, particularly if planting into a previous pasture or weedy fallow field.

Cucumber beetles
Larvae of cucumber beetles (rootworms) bore small shallow holes into the roots, similar to damage by wireworms. Cucumber beetle larvae are whitish with a brown head capsule, have three pairs of legs near the head, and are smaller (less than 1/3 inch) and softer bodied than wireworms (Figure 17). Two species of cucumber beetle attack sweet potato, the banded and the spotted cucumber beetles. Immatures of these species appear identical. Adults of the spotted cucumber beetle are yellowish- green with 12 black spots and adults of the banded cucumber beetle have yellowish and green bands across the body (Figure 18). Both species have similar biology with multiple generations per year. Adults feed on foliage, but their damage is minimal. Adults may be monitored to determine pest pressure. Eggs are laid in the soil.



Flea beetles
Flea beetle larvae feed on the roots of plants but show a preference for fibrous roots and thus are reported to only be of concern in sweet potato under high population densities. Feeding by larvae results in narrow winding tunnels on the surface of roots. Larvae are white with a brown head, soft-bodied, about 3/8 inch long and have a fleshy knob-like protuberance on the tail end. Multiple species can attack sweet potato and adults vary in color but are small beetles with an enlarged leg segment in the hind leg and jump when disturbed (thus the common name). Adults feed on foliage causing small shot-hole damage, which is of no consequence in production. Eggs are laid in the soil.
Whitefringed beetle
Larvae feed on the roots causing damage similar to white grubs. Larvae are yellowish-white with light brown heads (usually not visible), slightly curved and legless (Figure 19). Adults appear grayish with two longitudinal white bands along the side of the body. Larvae are reported to remain deep in the soil where preplant insecticides may not reach, making insecticidal control inconsistent at best. Adults are flightless, so rotation with nonhost crops (corn, sorghum, or grass cover crops) reduces potential problems with this pest. Fields with known populations should be avoided.


Sweet potato weevil
The sweet potato weevil is unique among sweet potato pests in that all stages can be found within the roots of sweet potato. It can attack the crop throughout its development, it can reproduce in storage, and it burrows tunnels throughout the root. The presence of this insect within the root causes a bitter taste. It is a regulated pest that causes restrictions on shipment of all plant parts into noninfested production areas.

Figure 20. Sweet potato weevil adult. Photo: Florida Department of Plant Industry, Florida Department of Agriculture and Consumer Services, Bugwood.org
Adults are distinctive in appearance. They are small (1/4 inch), thin-bodied weevils with the general appearance of a brightly colored ant (Figure 20). The head and wing covers are metallic dark blue and the thorax and legs are red-orange. Females bore shallow holes into stems and roots for oviposition and place a single egg per hole. The vast majority of oviposition occurs within 1 inch of the soil line, which contributes to the management recommendations listed below. Eggs hatch into small grubs (with dirty white, C-shaped, legless bodies and brown heads) that will burrow throughout roots leaving frass-filled tunnels. This feeding also triggers the production of terpene in the root, which imparts a bitter taste. Pupation and adult emergence also occurs within the root. All stages of weevil development occur within the root; thus, weevils can easily be moved into storage at harvest time and can reproduce within storage. Under good conditions, generation time is less than a month with multiple generations per year. The high potential for damage at even low densities and the potential ease of movement with the crop has resulted in sweet potato production regions that are free of the weevil and restrict shipment of sweet potato roots and foliage from infested areas. Shipment of roots into these areas generally requires fumigation, which is risky, as improper fumigation results in root death and rapid deterioration. Management of sweet potato weevil includes restrictions on slip production and cutting, field location, adult monitoring (with pheromone traps), and insecticide applications in field production and storage.
Foliar pests
Defoliating insects
Adults of several of the soil insect pests feed on foliage but are generally of minimal concern. Caterpillars are insect pests that have the potential to cause considerable defoliation to sweet potato. Species that commonly attack sweet potato include beet armyworm, looper, southern armyworm, variegated cutworm, and sweet potato hornworm. Defoliation impacts yield in a range from 30 to 50%, so growers should monitor defoliation and treat only as necessary to infrequently apply insecticides for defoliators.
Infestations of defoliating caterpillars at harvest time may require control actions even at relatively low pest levels. Destruction of foliage removes the host material for these caterpillars, and turning the roots onto the soil surface allows these insects to feed on the marketable produce. Large caterpillars can cause enough damage to render roots unmarketable and can be collected with roots and placed into storage where they will continue to feed until they reach the pupal stage. While caterpillars will not reproduce in storage, they can cause considerable damage. Fumigation will kill caterpillars but it may also damage the roots.
Aphids and whiteflies
Both aphids and whiteflies are reported as pests of sweet potatoes. While sweet potato whitefly is a severe pest of multiple vegetable crops in Georgia in the fall, it has not generally occurred in damaging populations in sweet potato. This pest does prefer hot, dry conditions, which are common during the sweet potato production season in Georgia. In recent years, whiteflies have developed damaging poulations in some areas, particularly after mild winters in Tift County and surrounding areas. Aphids, including the melon aphid and green peach aphid, may cause direct damage under high populations but are of greatest concern as potential vectors of viral diseases. Aphids and whiteflies have not been reported as consistent pest problems; however, if present they are not likely to be controlled by common management practices for soil pests. Please contact your county Extension agent if problems are encountered.
Managing Soil Pests of Sweet Potato
Managing soil pests starts with field site selection and preparation. Soil pests present at planting likely originated in the prior crop. Avoid growing sweet potatoes behind sweet potatoes (which particularly favors sweet potato weevil), corn, sod, or weedy fallow fields. These situations favor infestation of white grubs and wireworms, and those species with life cycles of a year or longer will still be present in the soil when sweet potatoes are planted. When planting in fields with a known infestation of soil insects, deep plowing three times prior to planting reduces infestations through direct mortality and exposure of insects to predators and parasites.
Management of sweet potato weevil requires even greater attention to field location, starting with plant beds. It is generally recommended that plant beds be located at least a mile from the prior year’s production fields and from fields to be planted from these beds. For weevil management, seed beds should be started with weevil-free seed pieces, treated weekly with an effective insecticide, and destroyed immediately after the final cutting. Cuttings should be made at least 1 inch above the soil surface (the vast majority of weevil eggs are laid within an inch of the soil surface) to avoid moving any weevil eggs into production fields.
While sampling methods and thresholds have been developed for some soil insect pests, the majority of producers use preventive soil-applied insecticides to manage these pests. Apply a broad-spectrum insecticide prior to planting to reduce populations of any soil pests present and to provide early season protection (three or more weeks) from reinfestation. These insecticides are incorporated to aid contact with the insects and to place the insecticide out of direct sunlight to avoid rapid breakdown. This preplant incorporated application is followed by a layby application of insecticide to establish a barrier against reinfestation by beetle larvae. Again, it is important to incorporate this application to form a barrier to soil penetration and place the insecticide out of direct sunlight. Research has shown that chemigation with the soil insecticide performed as well as mechanically incorporated insecticide applications. Additional foliar applications for soil insects are likely unnecessary for most species, but may well be justified if sweet potato weevil is present, particularly at harvest time. Sweet potato weevil pest pressure can be monitored with commercially available pheromone traps. Traps are baited with a synthetic sex pheromone, which attracts only male weevils. These traps can be used to monitor weevil activity in plant beds, storage houses and production fields.
Prevention of weevil damage to roots can also be aided by cultivation and adequate irrigation. Adult weevils oviposit into exposed roots and can reach the roots through cracks in the soil. Throwing soil around the base of plants with cultivation and irrigating to prevent soil cracking will reduce root exposure to weevil oviposition.
Foliar insecticide applications for soil pests
For sweet potato weevil, apply foliar insecticides on a 10-day schedule throughout the season. Insecticides applied for sweet potato weevil would also control adults of the other soil insect pest complex. The need for foliar insecticides is questionable when an adequate soil insecticide program is implemented. If relying on foliar insecticides for pest control, pest populations should be monitored at least weekly. Thresholds reported from Louisiana include two cucumber beetles per 100 sweeps (triggers weekly applications; apply on five-day schedule if higher populations occur), five flea beetles per 100 sweeps, or one whitefringed beetle per 100 sweeps. Weekly applications (again from Louisiana) are also triggered if any pheromone trap (three traps per each 40 acres) catches four weevils in one week. Attempts to ensure a weevil-free crop are likely to trigger at least weekly insecticide applications if any weevils are caught.
Additional insecticide applications
In years when whiteflies enter the crop early and have the potential to develop damaging populations, insecticide applications are warranted. A variety of insecticides are registered for whitefly in sweet potato, however, all are primarily active only on immature whiteflies. If whiteflies show the potential to develop damaging populations, insecticidal control should be implemented early in population development. At harvest, defoliating caterpillars may also require insecticidal control to prevent damage to tubers during and after harvest. This will require insecticide applications before terminating the crop and must be timed to allow time for caterpillar mortality and the proper preharvest interval for the insecticide.
Storage potatoes
Storage areas should be thoroughly cleaned and treated with insecticide prior to placing roots into storage. Seed potatoes should be collected from weevil-free fields. Roots placed into storage should be treated with an approved insecticide to prevent sweet potato weevil reproduction. All roots must be covered with insecticide to provide control.
Sweet potato weevil regulations
Georgia no longer runs a sweet potato weevil program, but growers are responsible for following regulations from other sweet potato-producing states if shipping potatoes into or through those states. In general, growers from Georgia cannot ship sweet potatoes into noninfested production regions unless the shipment has been fumigated (other restrictions also apply). Shipments of sealed containers through these regions are allowed. The Georgia Department of Agriculture has been in discussions with these states concerning potential adjustments to the requirements and should be contacted for information.
Weed control in sweet potato
Crop rotation, tillage, and a sound herbicide program are all critical components for long-term success. This publication focuses on developing sound herbicide programs while minimizing crop injury for transplant production systems. A new indemnified label for Dual Magnum has greatly improved weed control options, and growers must have these labels in hand at the time of application. It is critical for growers to understand that their specific production practices may alter weed and crop responses. Growers should evaluate these programs on limited acres until gaining experience.
Step 1: Fields must be weed-free when planting. Tillage, Roundup, and Gramoxone are all effective tools. For fields with a flush of difficult-to-control weeds prior to planting, apply Roundup (at the max rate for nutsedge) and then follow with Gramoxone five to seven days after the Roundup and at least a day prior to planting.
Step 2: Valor SX 51 WDG (up to 2.5 oz/acre) would improve weed control in nearly every Georgia field. It should be applied two to five days prior to transplanting to the preformed row. Do not incorporate and minimize movement of soil during transplanting. Do not apply after transplanting. The label notes to not use greenhouse- grown transplants and to test a small area for new cultivars.
Step 3: Command 3 ME (up to 1.5 pt/acre) should be applied post- transplant within five days of transplanting for preemergence control of annual grasses and a few broadleaf weeds. Roots must be below the surface where spray will occur. Command has great crop tolerance, but one must review the label regarding buffers and rotational restrictions.
Step 4: Dual Magnum can be used post as long as the applicator obtains the indemnified label prior to application (see back on steps to obtain label). Research has shown stunting from Dual Magnum if applied too closely to planting, so experimenting with applications two to three weeks after transplanting is suggested. Rates should range from 8-12 oz/acre. Sequential applications can be made as long as the total use rate does not exceed 1.33 pt/acre and applications are not made within 40 days of harvest.
Step 5: Select and Poast can be applied to control small annual grasses up until 30 days of harvest.
Critical points
No effective herbicide is currently available to control nutsedge. Ideally, avoid fields heavily infested with nutsedge. Maximum rates of glyphosate preplant and tillage are the most effective options.
Devrinol is labeled for sweet potato production fields and is effective on a few small-seeded broadleaf and grass weeds. Research has not noted a benefit when adding Devrinol to the program above.
Command poses serious carryover risks and has buffers. Check labels closely before use.
Be aware of potential carryover from previously used herbicides, especially Cadre.
- Do not apply Dual Magnum preplant or pre.
- Plowing is very effective. If plowing, follow immediately with a residual herbicide.
- Use conservative herbicide rates on sandy soils with low organic matter and/or with intense irrigation.
Successful weed management depends on residual herbicides that need to be activated by rainfall or irrigation within a day or two of application.
Always follow herbicide label restrictions and read labels for potential injury or carryover concerns.
References
Abel, C.A., Adams, L.C., & Stetina, S.R. (2007). Sweet potato yield reduction caused by reniform nematode in the Mississippi Delta. Plant Health Progress.
Ames, T., Smit, N.E.J.M., Braun, A.R., O’Sullvan, J.N., & Skoglum, L.G. (1997). Sweet potato: Major pests, diseases and nutritional disorders. International Potato Center, Peru, 104-111.
Chalfant, R.B., Bondari, K., Sumner, H.R., & Hall, M.R. (1992). Reduction of wireworm (Coleoptera: Elateridae) damage in sweet potato with insecticides applied by chemigation. Journal of Economic Entomology 86: 123-130.
Chalfant, R.B., Jansson, R.K., Seal, D.R., & Schalk, J.M. (1990). Ecology and management of sweet potato insects. Annual Review of Entomology 35: 157-180.
Clark, C.A., & Wright, V.L. (1983). Effect and reproduction of Rotylenchulus reniformis on sweet potato selections. Journal of Nematology 15: 198-203.
Clark, C.A., & Moyer, J.W. (1988). Compendium of sweet potato diseases. APS, St. Paul, Minnesota. 74.
Coolong, T., Seebold, K., Bessin, R., Woods, T., & Fannin, S. (2012). Sweet potato production for Kentucky. Univ. of Kentucky Cooperative Extension Bulletin-195. 16.
Gajanayake B., Reddy, K.R., Shankle, M.W., Arancibia, R.A., & Villordon, A.O. (2014). Quantifying storage root initiation, growth, and developmental responses of sweet potato to early season temperature. Agronomy Journal. 106:1795-1804.
Hammond, A. (2005). Sweet potato insect pest management (IPM). LSU AgCenter.
Heald, C.M., & Robinson, A.F. (1990). Survey of current distribution of Rotylenchulus reniformis in the United States. Journal of Nematology 22: 695- 699.
Jansson, R.K., Mason, L.J., Heath, R.R., Sorensen, K.A., Hammond, A.M., & Robinson, J.V. (1992). Pheromone-trap monitoring system for sweet potato weevil (Coleoptera: Apionidae) in the Southern United States: effects of trap type and pheromone dose. Journal of Economic Entomology 85: 416-423.
Jatala, P. (1991). Biology and management of plant-parasitic nematodes on sweet potato. In: Jansson, R.K. and Raman, K.V. eds. Sweet potato Pest Management, A Global Perspective. Westview Press, Boulder, CO. 359-378.
Johnson, A.W., Dowler, C.C., Glaze, N.C., & Handoo, Z.A. (1996). Role of nematodes, nematicides, and crop rotation on the productivity and quality of potato, sweet potato, peanut, and grain sorghum. Journal of Nematology 28: 389-399.
La Bonte, D.R., Wilson, P.W., Villordon, A.Q., & Clark, C.A. (2008). ‘Evangeline’ sweet potato. HortScience 43: 258-259.
McSorley, R. (1980). Nematodes associated with sweet potato and edible aroids in southern Florida. Proceedings of Florida State Horticultural Society 93: 283-285.
Overstreet, C. (2009). Nematodes. In: Loebenstein, G., and Thottappilly, G., eds. The sweet potato. Springer: the Netherlands. 135-159.
Rubatzky, V., & Yamaguchi, M. (1997). World vegetables- Principles, production, and nutritive values 2nd Ed. Chapman and Hall, New York. 843.
Scott, L.E., & Bouwkamp, J.C. (1974). Seasonal mineral accumulation by the sweet potato. HortScience 9:233-235.
Scurrah, M., Niere, B., & Bridge, J. (2005). Nematode parasites of potato and sweet potato. In: Luc, M., Sikora, R., Bridge, J., eds. Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd edn. CAB International: Wallingford, UK. 193-221.
Seal, D.R., Chalfant, R.B., & Hall, M.R. (1992). Effects of cultural practices and rotational crops on abundance of wireworms (Coleoptera: Elateridae) affecting sweet potato in Georgia. Environmental Entomology. 21: 969-974.
Seal, D.R., Chalfant, R.B., & Hall, M.R. (1992). Effectiveness of different seed baits and baiting methods for wireworms (Coleoptera: Elateridae) in sweet potato. Environmental Entomology 21: 957-963.
Smith, T.P., & Beuzelin, J.M. (2014). Insect pest management in Louisiana Sweet potatoes. LSU AgCenter Pub. 2620. 8.
Thomas, R.J., & Clark, C.A. (1983). Population dynamics of Meloidogyne incognita and Rotylenchulus reniformis alone and in combination, and their effects on sweet potato. Journal of Nematology 15: 204-211.
Yencho, G.C., Pecota, K.V., Shultheis, J.R., VanEsbrock, Z.P., Holmes, G.J., Little, B.E., Thornton, A.C., & Truong, V.D. (2008). ‘Covington’ sweet potato. HortScience 43: 1911-1914.
Wang, K.H., Sipes, B.S., & Schmitt, D.P. (2001). Suppression of Rotylenchulus reniformis by Crotalaria juncea, Brassica napus, and Target erecta. Nematropica 31: 237-251.
Zehnder, G. (1998). A sweet potato grower’s guide to insect pest management. Alabama Cooperative Extension System ANR-1104. 6.
Zhang, S., Zhang, S., Wang, H., & Chen, Y. (2006). Characteristics of sweet potato stem nematode in China. Acta Phytopathologica Sinica 36: 22-27.
Status and Revision History
Published on May 03, 2018
Published with Minor Revisions on Oct 30, 2018
Published with Full Review on Apr 21, 2023
