Wheat leaf rust, caused by the fungus Puccinia triticina (formerly known as Puccinia recondita f. sp. tritici [Figure 1]), is often a destructive foliar disease of wheat in the state of Georgia. Rust fungi in wheat are highly specialized pathogens with narrow host ranges.
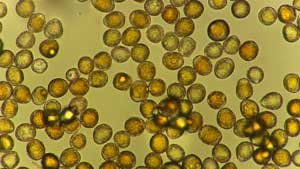
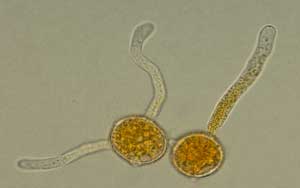
Figure 1. Micrographs of urediniospores (left) and germinating urediniospores (right) of Puccinia triticina, the causal agent of wheat leaf rust. (Photos: Alfredo Martinez, James Buck; taken using 40X and 100 X objectives.)
Symptoms



Figure 2. Symptoms of wheat leaf rust. (Photos: Andrew Sawyer and Alfredo Martinez.)
Flaky, reddish-brown, 1/16-in. (1.5 mm) in diameter pustules develop on leaves and sheaths (Figure 2). Pustules break through the leaf epidermis, and spores are easily dislodged by rain, wind, or contact. Pustules may be found early in the growing season on lower leaves, but they often appear in large numbers on the upper leaves after flowering. A single spore can invade a leaf and produce a pustule with thousands of new spores within 7 to 10 days. This allows the disease to spread rapidly and cause extensive damage within a short time.
Conditions Favoring the Disease
High relative humidity and/or free moisture and temperatures ranging from 59 °F to 77 °F (15 °C to 25 °C) are conducive for leaf rust to develop (Table 1). The optimum temperature for urediniospore germination is 68 °F (20 °C). If these conditions exist, infection can occur in 6 to 8 hr. Leaf rust epidemic severity increases exponentially over time. Dry, windy days, which disperse spores followed by cool nights with dew, also favor leaf rust epidemics. Urediniospores serve as primary inoculum by virtue of long distance dispersal by wind.
| Table 1. Environmental Conditions Required for Leaf Rust Growth Stages | |||||
| Leaf rust stage | Temperature (°F/°C) | Light | Free Water | ||
| Minimum | Optimum | Maximum | |||
| Germination | 35/2 | 68/20 | 86/30 | Low | Essential |
| Germling | 41/5 | 59-68/15-20 | 86/30 | Low | Essential |
| Appressorium | - | 59-68/15-20 | - | None | Essential |
|
Penetration |
50/10 | 68/20 | 86/30 | No effect | Essential |
| Growth | 35/2 | 77/25 | 95/35 | High | None |
| Sporulation | 50/10 | 77/25 | 95/35 | High | None |
| Modified from R.P. Singh, J. Huerta-Espino, A.P. Roelfs. 2002. | |||||
Control
Several methods can be used to prevent, manage and treat wheat leaf rust.
Genetic Control
 Figure 3. Screening of wheat germplasm at CAES Research and Education station in Plains, GA. (Photo: Alfredo Martinez.)
Figure 3. Screening of wheat germplasm at CAES Research and Education station in Plains, GA. (Photo: Alfredo Martinez.)Use of resistant varieties is the best way to control wheat losses to leaf rust. Resistant varieties have one or more specific leaf rust resistance genes (denominated Lr genes). There are more than 30 different Lr genes available to date; however, most varieties have only a few Lr genes. In order to cause disease (i.e., be virulent) on a certain variety, the leaf rust fungus must be able to defeat all the Lr genes in that variety. The prevalence of different rust races is always changing in response to the different wheat varieties being grown with different Lr genes. Because new races of the fungus can develop, it is important to know the susceptibility of a given wheat variety. Table 2 lists the relative rust resistance of the wheat varieties recommended for Georgia in 2014–15. See Figure 3 for an example of wheat germplasm screening in Georgia.
| Table 2. Leaf Rust Resistant Wheat Variety Recommendations | ||
| GOOD | FAIR | POOR |
| AGS 2026 | Dyna-Gro 9171 | Pioneer 26R20 |
| AGS 2027 | Pioneer 26R10 | Roberts |
| AGS 2035 | Pioneer 26R61 | TV8525 |
| AGS 2038 | SS 8415 | TV8848 |
| AGS 2060 | SS 8629 | USG 3555 |
| Dyna-Gro Baldwin | TV8535 | |
| Fleming | TV8861 | |
| Jamestown | ||
| LA754 | ||
| Oglethorpe | ||
| Pioneer 26R94 | ||
| SS 8641 | ||
| USG 3024 | ||
| Source: Georgia 2013–2014 Small Grains Performance Tests, 禁漫天堂 Extension Annual Publication No. 100-6, /publications/detail.html?number=AP100-6. | ||
Field Monitoring
 Figure 4. Rust spores on shoes after walking through a heavily infected field. (Photo: John Youmans.)
Figure 4. Rust spores on shoes after walking through a heavily infected field. (Photo: John Youmans.)Check fields periodically during the season, especially when warmer temperatures start to develop. Use a hand lens to look for symptoms on plant leaves and examining a number of plants throughout the field. Spores of rust are often seen on the upper portion of the leaf. Field confirmation of rust spores can be made by rubbing your fingers over the top of the leaf. Spores appear as a red-brown, clay matter on your fingers. Infection usually starts at the bottom of the plant and moves up. Usually field symptoms are so conspicuous and unique that visual inspection will suffice for a rapid identification (Figure 4). However, if symptoms are not sufficient to identify the disease, then a physical sample might be needed for identification. Information on how and where to submit a sample is located at or by calling your county Extension office (1-800-ASK-禁漫天堂1).
Cultural Control
It is recommended to implement practices that include the eradication of volunteer plants and crop debris, which can harbor inoculum over the winter. This cultural practice does not guarantee freedom from rust because urediniospores are carried long distances by wind. Avoid early sowing and excess nitrogen applications.
Chemical Control
 Figure 5. Evaluation of chemical options for control of leaf rust at the CAES Research and Education Center in Plains, GA. (Photo: Alfredo Martinez.)
Figure 5. Evaluation of chemical options for control of leaf rust at the CAES Research and Education Center in Plains, GA. (Photo: Alfredo Martinez.)There are several fungicides labeled for leaf rust on wheat. Generally there is no economic benefit from applying fungicides to control leaf rust when resistant varieties are grown. However, exceptions can occur. There are only a few varieties that are highly resistant to leaf rust, and there is some potential for use of fungicides. Possible disease race shifts may make it necessary to use fungicides. IF leaf rust is found in the field, a fungicide application is recommended. Protection of the flag leaf is critical for yield preservation. Due to constant changes in fungicide labeling, check the entire product label and/or contact your local county Extension agent for the most up-to-date information. Fungicides for managing leaf rust are found in Table 3. Additional information is found in the Georgia Pest Management Handbook (禁漫天堂 Extension Special Bulletin 28 — ). Always follow product labels for recommendations, precautions, and restrictions. See Figure 5 for an example of fungicide efficacy evaluations for wheat leaf rust control in Georgia.
| Table 3. Fungicides for Leaf Rust of Wheat2 | |||||
| Fungicide(s) | Leaf Rust1 |
Harvest Restriction |
|||
| Class | Active Ingredient | Product | Rate/acre (fl oz) |
||
| Strobilurin | Picoxystrobin 22.5% | Aproach SC | 6.0–12 | VG | Feekes 10.5 and 45 days |
| Fluoxastrobin 40.3% | Evito 480 SC | 2.0–4.0 | VG | Feekes 10.5 and 40 days |
|
| Pyraclostrobin 23.6% | Headline SC | 6.0–9.0 | E | Feekes 10.5 | |
| Triazole | Metconazole 8.6% | Caramba 0.75 SL | 10.0–17.0 | E | 30 days |
| Propiconazole 41.8% | Tilt 3.6 EC3 | 4.0 | VG | Feekes 10.5 | |
| Prothioconazole 41% | Proline 480 SC | 5.0–5.7 | VG | 30 days | |
| Tebuconazole 38.7% | Folicur 3.6 F3 | 4.0 | E | 30 days | |
| Prothioconazole 19% Tebuconazole 19% |
Prosaro 421 SC | 6.5–8.2 | E | 30 Days | |
| Mixed modes of action |
Metconazole 7.4% Pyraclostrobin 12% |
TwinLine 1.75 EC | 7.0–9.0 | E | Feekes 10.5 |
| Fluxapyroxad 14.3% Pyraclostrobin 28.6% |
Priaxor | 4.0–8.0 | VG | Feekes 10.5 | |
| Propiconazole 11.7% Azoxystrobin 7.0% |
Quilt 200 SC3 | 10.5–14.0 | E | Feekes 10.5 | |
| Propiconazole 11.7% Azoxystrobin 13.5% |
Quilt Xcel 2.2 SE | 10.5–14.0 | E | Feekes 10.5 | |
| Prothioconazole 10.8% Trifloxystrobin 32.3% |
Stratego YLD | 4.0 | VG | Feekes 10.5 35 days |
|
| Tebuconazole 22.6% Trifloxystrobin 22.6% |
Absolute 500 SC | 5.0 | E | 35 days | |
| Cyproconazole 7.17% Picoxystrobin 17.94% |
Aproach Prima SC | 3.4–6.8 | VG | 45 days | |
| 1 Efficacy categories: VG = Very Good; E = Excellent. 2 Modified from 2014 fungicide table produced by "The North Central Regional Committee on Management of Small Grain Diseases (NCERA-184)" and from the CAES Wheat Production Guide (). This information is provided only as a guide. By law, it is the responsibility of the pesticide applicator to read and follow all current label directions. No endorsement is intended for any products listed, nor is criticism meant for products not listed. 禁漫天堂 and members or participants in the NCERA-184 committee assume no liability resulting from the use of these products. |
|||||
References
Agrios, G. N. (2005). Plant pathology (5th ed.). Elsevier Academic Press Inc.
Bockus, W. W., Bowden, R. L., Hunger, R. M., Morrill, W. L., & Murray, T. D. (Eds.). (2010). Compendium of wheat diseases and pests (3rd ed.). Amer Phytopathological Society.
Bowden, R. L. (2000). Wheat leaf rust. http://www.plantpath.ksu.edu/doc768.ashx
Buntin, D. G., & Cunfer, B. M. (Eds.). (2000). Southern small grains resource management handbook (Publication No. B1190). 禁漫天堂 Cooperative Extension.
DeWolf, E. (2014). NCERA-184 Management of small grain diseases. Kansas State University.
Kolmer, J. (2013). Leaf rust of wheat: Pathogen biology, variation and host resistance. Forests, 4, 70–84. https://doi.org/10.3390/f4010070
Martinez-Espinoza, A. (2014). Disease management in wheat. In 2013-2014 wheat production guide. http://www.caes.uga.edu/commodities/fieldcrops/gagrains/documents/2013-14WheatProductionGuide.pdf
Singh, R.P., Huerta-Espino, J., & Roelfs, A.P. (2002). The wheat rusts. In B. Curtis, S. Rajaram, & H. Macpherson (Eds.), Bread wheat: improvement and production (FAO Plant Production and Protection Series No. 30). Food and Agriculture Organization of the United Nations.
Strand, L. (1990). Integrated pest management for small grains (UC ANR Publication No. 3333). University of California Division of Agriculture and Natural Resources.
Ulloa, M., & Hanlin, R. T. (2012). Illustrated dictionary of mycology. (2nd ed.). Amer Phytopathological Society.
Status and Revision History
Published on Nov 17, 2014
Published with Full Review on Jun 17, 2022
