- Causal organism
- Disease cycle and causal conditions
- Symptoms
- Cultural controls
- Chemical controls
- References
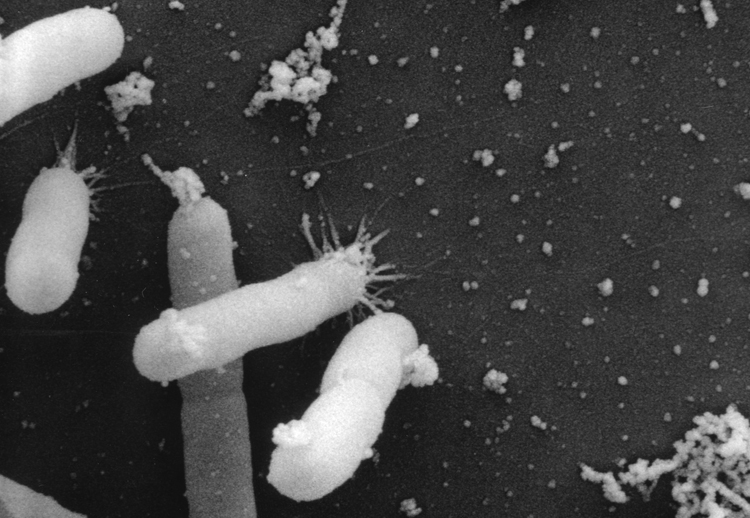 Figure 1. Electron microscope photograph of Xylella fastidiosa bacteria (Pierce's disease of grape isolate) attached to a membrane surface. The bacteria and associated bacterial and plant-associated products will clog the xylem elements, the conductive tissues that transport water and nutrients from the roots, causing scorch symptoms and eventual plant death. Photo by H.C. Hoch, Cornell University; Journal of Bacteriology 189:7507-7510.
Figure 1. Electron microscope photograph of Xylella fastidiosa bacteria (Pierce's disease of grape isolate) attached to a membrane surface. The bacteria and associated bacterial and plant-associated products will clog the xylem elements, the conductive tissues that transport water and nutrients from the roots, causing scorch symptoms and eventual plant death. Photo by H.C. Hoch, Cornell University; Journal of Bacteriology 189:7507-7510.Relative to total sales, blueberries are the number one fruit commodity in the state of Georgia, surpassing even peaches. Recently, a new disease has been identified in the Georgia blueberry production region. This disease has been named "bacterial leaf scorch," and it is caused by the bacterium Xylella fastidiosa (Figure 1).
Koch's postulates were recently utilized to confirm this new disease; the suspected disease-causing agent (bacterium) was isolated from a diseased plant, cultured, and reintroduced into a healthy plant in which subsequent disease symptoms developed that were similar to the original plant. For bacterial leaf scorch, Koch's postulates were conducted by Dr. C.J. Chang (University of Georgia) in the summer of 2006, and they were fulfilled in November 2006.
Through initial field surveys conducted in the summer of 2007, it was determined that this disease has the potential to become a major threat to blueberry production in Georgia and elsewhere, especially in the southern highbush blueberry varieties. Among these varieties, 'FL86- 19' (alias 'V1') has proven to be the most susceptible. However, 'Star' and other varieties are also showing substantial disease incidence and severity in several locations. At this early stage, little is known for sure about the epidemiology (means of dissemination and spread) of this disease, and the basic research to determine the means of spread and interaction within the south Georgia environment needs to be completed. In addition, research-based control methods need to be established for this disease. Current recommendations are based on information derived from other plant systems, such as wine grapes, and information needs to be developed specifically for blueberries.
Causal organism
The Xylella fastidiosa bacterium is pathogenic on numerous plant species. In addition, it is known to inhabit many host plants without causing disease symptoms; among these are various grasses and herbaceous weeds that are generally found throughout the blueberry production region of Georgia. Native blueberries also likely harbor the bacterium; therefore, there is generally a bacterial reservoir readily available for infection.
There are Xylella subspecies, of which X. fastidiosa subsp. fastidiosa and subsp. multiplex are major. The Xylella that causes Pierce's disease of grape falls in the fastidiosa subspecies, while the Xylella that causes phony peach falls in the multiplex subspecies. Both Pierce's disease and phony peach are examples of other major Xyllella-incited diseases that also occur in Georgia. The specific Xylella fastidiosa strains found in blueberry are generally unique recombinant multiplex strains. However, recent reports have indicated that some fastidiosa strains can also infect and cause symptoms on blueberry
As in other Xylella-incited diseases, it is assumed that the bacterium blocks xylem vessels, thereby preventing water and nutrient flow from the soil throughout the plant. This bacterium only survives in plant xylem or within the insects that vector it. In general, Xylella diseases are more prevalent in warmer environments; this is related in part to the fact that the insect vectors, primarily sharpshooters, survive better in warmer climates, but the bacterium also overwinters more successfully within host plants in warmer climates. Though it is speculation at this point, the increase in bacterial leaf scorch in Georgia may be at least partially related to warmer winters, which may have aided survival of vectors and the bacterium. South Georgia and Florida provide ideal environments for both the bacterium and the vectors to survive.
To date, the disease has been an obviously observed field problem of southern highbush blueberry varieties only. It is not known whether this disease is also causing chronic or acute problems in rabbiteye varieties, but the bacterium can in fact colonize rabbiteye plants. However, there is no doubt at this point that the disease is causing chronic and acute losses in southern highbush varieties
Disease cycle and causal conditions
Disease cycle and causal conditions The disease cycle of this bacterium in grape, peach, and plum is well known, and it is likely the same in blueberry. Infected hosts serve as reservoirs and overwintering sites of the bacterium. In the spring and early summer, insect vectors (sharpshooters and spittle bugs) transmit the bacterium by feeding on infected plant tissues and subsequently feeding on healthy plants. In other systems, the glassy-winged sharpshooter, Homalodisca vitripennis, is the most important vector, and 97 percent of the sharpshooters found in southern highbush blueberry plantings are glassy-winged sharpshooters (M. Tertuliano; personal communication). The glassy-winged sharpshooter can be found abundantly in south Georgia and Florida, where it is known to be the major vector of Xylella in peach and also prevents production of European wine grapes. Once the insect has acquired the bacterium, it is transmitted to a new plant as the insect injects the bacterium into the xylem (the conductive tissues that transmit water and nutrients from the roots to the other plant tissues) during feeding.
This bacterial species is unique in that it is limited to life in the plant xylem. Movement of the bacterium occurs throughout the plant xylem system, and movement to the roots is relatively rapid in blueberry—rendering pruning of symptomatic tissues ineffective. At some point, bacteria form colonies, and through a combination of tyloses, gumming, and bacterial exudate production, the xylem is clogged. In time, clogging of vessels reaches a point at which individual stems or whole plants will no longer be able to carry sufficient water and nutrients to support life. At this point symptoms develop, and eventually the plant will die. Plant death can be relatively rapid, but in general, symptom development starts in one year and continues through at least a second season before plant death.
This bacterium can also be transmitted through propagation from infected plants. Propagation studies have shown that apparently healthy cuttings taken from infected plants will sometimes produce diseased transplants, and a massive number of plants can be disseminated rapidly, expanding the epidemic. The combination of propagation and insect vectoring could cause rapid spread throughout the entire region. However, it is unlikely that symptomatic plants would be used for propagation.
Root grafting may also serve as a potential transmission mechanism. In high-density beds, this may be particularly important as a means of spread, but there has been no research conducted to date to support this premise.
Symptoms
 (2a)
(2a)  (2b)
(2b)  (2c)
(2c)  (2d)
(2d)Figure 2. Scorch symptoms (late summer) observed on plants infected with Xylella fastidiosa. In some cases, the marginal leaf burn is very distinct and surrounded by a dark line of demarcation between green and dead tissue (2a and 2b). In other cases, the symptoms are very similar to those of anthracnose leaf spot (2c); in fact, disease-causing and saprophytic organisms do opportunistically infect the marginal dead tissue, further confusing accurate identification. Sometimes, the scorched leaf area is bordered by a darker band between the healthy and scorched tissue, and on occasion an “oak-leaf” pattern will be observed (2d) (the green tissue that remains looks like an oak leaf surrounded by brown tissue).
The initial symptom is a marginal leaf scorch (burn), which unfortunately is similar to that observed with extreme drought, fertilizer salt burn, or root rots (Figure 2). Sometimes, the scorched leaf area is bordered by a darker band between the healthy and scorched tissue, and on occasion an "oak-leaf" pattern will be observed (green tissue that looks like an oak leaf surrounded by brown tissue). This leaf symptom can be uniformly distributed throughout the plant, but in the early stages scorching may be limited to individual stems or perhaps one side of the plant—indicating that only a partial xylem blockage has occurred and may be limited to one cane or one stem. Spring growth is characterized by twigs of very thin diameter. Eventually, leaves abscise (drop) and young twigs/stems may yellow (Figures 3-4). After leaf drop, the plant eventually dies (Figure 5). Often, where a plant has died from bacterial leaf scorch, a neighboring plant will show symptoms the following year.
The most unique symptom of bacterial leaf scorch is actually observed once leaves have dropped—the yellowed stems and twigs. The plant can drop virtually all leaves and yet remain otherwise healthy in appearance; the stems and root systems appear sound, and vascular discoloration is not generally observed. Once the leaves have dropped, the plant takes on a skeleton-like appearance. Dieback is not generally associated with this disease in the early stages; of course, the final result is plant death, and at that point it is not possible to diagnose the cause of death.
The bacterium cannot be easily observed with a light microscope, so confirmation is only possible through ELISA or PCR techniques in a lab. In addition, observation of bacterial growth on specialized media is also recommended for confirmation of this disease.

 Figure 3. Scorch symptoms (late summer) observed on plants infected with Xylella fastidiosa. The symptom observed here is the best indicator that the plants are actually dying of bacterial scorch as opposed to root rot, anthracnose, fertilizer salt or chemical injury, or simple drought stress, any of which can mimic bacterial leaf scorch. Prior to complete plant death, all leaves abscise (fall off) and the remaining stems take on a yellow, “skeletal” appearance. The root system and stems do not show any obvious lesions or dieback symptoms, and the plant will generally appear healthy, with the exception of complete defoliation.
Figure 3. Scorch symptoms (late summer) observed on plants infected with Xylella fastidiosa. The symptom observed here is the best indicator that the plants are actually dying of bacterial scorch as opposed to root rot, anthracnose, fertilizer salt or chemical injury, or simple drought stress, any of which can mimic bacterial leaf scorch. Prior to complete plant death, all leaves abscise (fall off) and the remaining stems take on a yellow, “skeletal” appearance. The root system and stems do not show any obvious lesions or dieback symptoms, and the plant will generally appear healthy, with the exception of complete defoliation. Figure 4. Panoramic view of an individual plant with bacterial scorch symptoms. Some surrounding plants are also starting to show early symptoms.
Figure 4. Panoramic view of an individual plant with bacterial scorch symptoms. Some surrounding plants are also starting to show early symptoms.
 Figure 5. Plants that have died from bacterial leaf scorch. At this stage, it would be very difficult to determine that bacterial leaf scorch was the cause of death, especially once plants have been hedged. Symptoms are virtually identical to those of root rot, but in this case, there is no excessive moisture or low areas that would be often associated with root rot. Also, the pattern of spread is different from root rot in that scattered dead plants are observed; root rot mortality is often clumped, especially around low, wet areas.
Figure 5. Plants that have died from bacterial leaf scorch. At this stage, it would be very difficult to determine that bacterial leaf scorch was the cause of death, especially once plants have been hedged. Symptoms are virtually identical to those of root rot, but in this case, there is no excessive moisture or low areas that would be often associated with root rot. Also, the pattern of spread is different from root rot in that scattered dead plants are observed; root rot mortality is often clumped, especially around low, wet areas.Cultural controls
 Figure 6. Resistance. In this planting, a single row of 'V5' plants was alternately planted after 10 rows of 'FL86-19' plants (repeated numerous times). The surrounding 'FL86-19' plants were all infected, with significant mortality, and they have been removed at this point. The 'V5' plants consistently showed no symptoms of disease or mortality after five years at this site. This indicates field resistance in the 'V5' line.
Figure 6. Resistance. In this planting, a single row of 'V5' plants was alternately planted after 10 rows of 'FL86-19' plants (repeated numerous times). The surrounding 'FL86-19' plants were all infected, with significant mortality, and they have been removed at this point. The 'V5' plants consistently showed no symptoms of disease or mortality after five years at this site. This indicates field resistance in the 'V5' line.(1) It is essential that new plants not be propagated from Xylella-infected plants. At this point, there is no testing program for propagation. However, propagators should never take cuttings from plants they have not personally inspected for visual disease symptoms. Diseased plants should never be used for propagation, whether they have symptoms of Xylella or other viruses or diseases of blueberry.
(2) Identification of Xylella-infected plants is possible in the field, and once such plants are identified, they should be flagged and immediately destroyed. By doing this, it is hoped that the epidemic will be slowed.
(3) There may also be a tie-in between plant stress and successful infection by Xylella; therefore, reduction of plant stresses, such as drought stress, may at least slow symptom development, if not preventing it altogether.
(4) There is varietal resistance in some southern highbush blueberries. The 'FL86-19' variety is particularly susceptible to infection and disease development by Xylella. When compared with other southern highbush or rabbiteye varieties, the 'FL86- 19' variety quickly develops symptoms and high bacterial titers after manual inoculations, which correlates well with observed susceptibility in the field. On the other hand, 'V5' has resistance to this bacterium (Figure 6). This is encouraging, since it indicates that breeding can be used to develop varieties that are highly resistant to Xylella. Likewise, surveys have shown that there are other varieties that either do not develop symptoms or that slow epidemic spread of the disease (Figure 7).
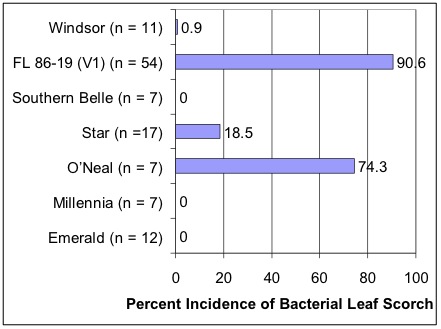 Figure 7. Incidence (percentage of symptomatic plants) of bacterial leaf scorch by cultivar at one site. The number of rows surveyed (n) is shown in parentheses next to the cultivar name. 'FL 86-19' is highly susceptible, as is the 'O'Neal' cultivar. 'Star' is susceptible, but it is representative of desirable cultivars that will develop the disease but still likely be economically viable; field epidemics observed in 'Star' and similar cultivars do not develop as rapidly, allowing adequate time to recoup investments.
Figure 7. Incidence (percentage of symptomatic plants) of bacterial leaf scorch by cultivar at one site. The number of rows surveyed (n) is shown in parentheses next to the cultivar name. 'FL 86-19' is highly susceptible, as is the 'O'Neal' cultivar. 'Star' is susceptible, but it is representative of desirable cultivars that will develop the disease but still likely be economically viable; field epidemics observed in 'Star' and similar cultivars do not develop as rapidly, allowing adequate time to recoup investments.
Chemical controls
At this point, there are no chemical controls that actively kill the bacterium. However, it may be possible to slow or even break the disease cycle by vector management, killing the insects that transmit the bacterium. There are several insecticides that are active against leafhoppers, and several of these are registered for use on blueberries.
(1) Application of soil-applied neonicotinoid products (imidacloprid or thiamethoxam) should take place as plants begin their initial spring flush. With any of the neonicotinoid materials, their systemic qualities will be best observed when there is good moisture and active growth. In parallel fashion, neonicotinoid longevity is much better with soil application than with foliar application. Product longevity and performance, based on results from ornamentals, are influenced by rate, so applying the highest labeled rate is best. For blueberries, the neonicotinoids labeled for soil application include imidacloprid products (Admire 2F, Admire Pro, Advise 2FL, Alias 2F, Courage 2F, Imida E-AG 2F, Nuprid 2F), and a thiamethoxam product (Platinum 2EC).
(2). During the late spring period, which may correlate with the period of actual vectoring by sharpshooters, foliar-applied pyrethroids and organophosphates should be used to augment or complement the neonicotinoid drench, especially if glassy-winged sharpshooters are observed in sticky traps.
As mentioned throughout this publication, additional research is needed to address the basic questions we have relative to this new menace to the blueberry industry. However, it is likely that this disease will mimic similar diseases in other plant systems. As such, we can conclude that a management program that includes cultural and chemical control and breeding programs is needed. Southern highbush blueberry producers should actively incorporate suggested management practices for this disease, as they have with others. Otherwise, the epidemic will likely increase throughout the region, resulting in major losses.
Contact your local county agent for additional information or see the current edition of the Georgia Pest Management Handbook for specific chemical recommendations.
References
Almeida, R. P. P., & Nunney, L. (2015). How do plant diseases caused by Xylella fastidiosa emerge. Plant Disease, 99(11), 1457–1467.
Chang, C. J., Donalson, R., Brannen, P., Krewer, G., & Boland, R. (2009). Bacterial leaf scorch, a new blueberry disease caused by Xylella fastidiosa. HortScience, 44(2), 413–417.
Holland, R. M. (2013). Location, transmission, and impact of Xylella fastidiosa in southern highbush blueberries [Master’s thesis, University of Georgia].
Meyer, M. M., & Kirkpatrick, B. C. (2007). Effects of cold temperatures and variety on cold curing of Xylella fastidiosa infected grapevines (Abstr.). Phytopathology, 97(7), S76.
Nunney, L., Hopkins, D. L., Morano, L. D., Russell, S. E., & Stoutthamer, R. (2014). Interspecific recombinations in Xylella fastidiosa strains native to the United States: Infection of novel hosts associated with an unsuccessful invasion. Appl. Environ. Microbiol., 80, 1159–1169.
Oliver, J. E., Cobine, P. A., & De La Fuente, L. (2015). Xylella fastidiosa isolates from both subsp. multiplex and fastidiosa cause disease on southern highbush blueberry (Vaccinium sp.) under greenhouse conditions. Phytopathology, 105, 855–862.
Varela, L., Smith, R. J., & Phillips, P. A. (2001). Pierce’s disease (Publication No. 21600). University of California Agricultural and Natural Resources.
Wells, J. M. (1995). Phony Peach. In J. M. Ogawa, E. I. Zehr, G. W. Bird, D. F. Ritchie, K. Uriu, & J. K. Uyemoto (Eds.), Compendium of stone fruit diseases (pp. 53–54). APS Press.
1½ûÂþÌìÌà Extension Plant Pathologist
2½ûÂþÌìÌà Extension Horticulturist
3½ûÂþÌìÌà Extension County Agent
4½ûÂþÌìÌà Extension Entomologist
5½ûÂþÌìÌà Plant Pathologist
Status and Revision History
Published on Feb 13, 2008
Published with Minor Revisions on Feb 11, 2011
Published with Minor Revisions on Jan 21, 2016
Published with Full Review on Oct 10, 2022
