Uttam Saha1
Leticia Sonon1
Jake Mowrer1
Dennis Hancock2
Lawton Stewart3
Nicholas Hill2
David Kissel1
Introduction
Selenium (Se) is one of at least 17 essential minerals needed in animal diets. Selenium is a structural component of several vital enzyme systems, such as glutathione peroxidase, that play vital roles in animal and human physiology and health. Though it is an essential micronutrient in animal and human diets, Se is also toxic to animals and humans when too much is consumed; therefore, it merits special attention in animal feeding to ensure that the concentration range is appropriate.
This publication highlights the following:
- The role of Se in animal nutrition;
- Se concentration and distribution in soils and feedstuffs (grains and forages) produced in various parts of the United States and in Georgia;
- Disorders resulting from Se deficiency or toxicity;
- Various methods of Se supplementation; and
- Recommendations for Se management in Georgia.
This publication is intended to serve as an educational resource for university researchers and Extension specialists, county Extension agents and livestock, forage and feed producers, among others.
Functions of Selenium
In normal animal and human metabolism, oxidation breaks down (or burns) carbohydrates, fats and proteins from feeds or foods to produce carbon dioxide, water and energy. This energy is utilized in various body functions such as work, weight gain, milk production, etc. However, oxidation of a body's structural (e.g., cell membranes) and functional (e.g., enzymes and intracellular substances) components is very harmful. A simple analogy is that gasoline is burned to drive a car, but it is also necessary to protect the car's components from the extreme heat of combustion. The body must also have a defense mechanism to protect its components from oxidation-induced damage. This defense is provided by an antioxidant defense mechanism, or antioxidant activity.
Water is produced by reduction (the opposite of oxidation) of molecular oxygen. The process of sequential reduction of molecular oxygen to water leads to the formation of the following reactive oxygen species (ROS) in sequence:
- superoxide anion (which is both an ion and a radical)
- peroxide (hydrogen peroxide; organic peroxides)
- hydroxyl radical (the most reactive of all)
Figure 1 provides further information about these ROS. In addition to ROS, breakdown of proteins results in the formation of nitrogen free radicals (NFRs) like nitric oxide (NO).
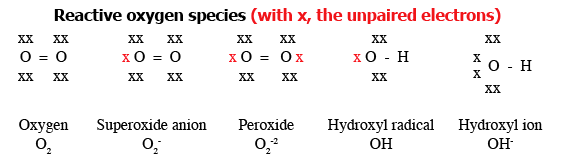 Figure 1.
Figure 1. Various reactive oxygen species§ that can form in sequence during reduction¶ of molecular oxygen. [Adapted from Bowen (2003).
Available online at: http://www.vivo.colostate.edu/hbooks/pathphys/misc_topics/radicals.html (accessed May/24/2011).]
§Reactive oxygen species (ROS) are ions or very small molecules that include superoxide anion, free radicals and peroxides (both inorganic and organic). They are highly reactive due to the presence of unpaired valence shell electrons.
¶Equivalent to sequential addition of electrons.
Note: The hydroxyl radical differs from the hydroxyl ion; the neutral form is a hydroxyl radical and the hydroxyl anion actually carries a negative charge.
Both ROS and NFRs are powerful oxidizing agents. If ROS and NFR are not destroyed, they can damage living cells by attacking their proteins, lipids (fat) and nucleic acids (e.g., DNA). Unsaturated fatty acids, which are the major component of all cell membranes, are particularly susceptible. Their oxidation by ROS results in the formation of lipid hydroperoxides (organic peroxides), which are quite damaging. Membranes particularly at risk of such damage include those in mitochondrial, red blood and gastrointestinal cells.
The glutathione peroxidase family of enzymes helps prevent the formation of ROS and NFR. They also destroy the hydrogen peroxide (by converting it to water) and lipid hydroperoxides (by converting them to lipid alcohols), thus preventing oxidative damage and ensuring cell membrane integrity. Because Se is an integral component of glutathione peroxidase, an adequate level of Se in the animal body is essential for maintaining proper levels of the enzymes. Selenium deficiency decreases the activity of glutathione peroxidase and other Se-dependent enzymes involved in antioxidant activity, thereby reducing the body's defenses against oxidative damage.
Interaction of Selenium with Vitamin E and Other Nutrients
Several vitamins, including vitamin E, are important in human and animal nutrition. Some vitamins are soluble in water whereas others are soluble in fat (or lipid). Vitamin E is fat soluble, whereas Se is water soluble. Both Se and vitamin E reduce oxidative damage as follows:
- Vitamin E blocks ROS attacks on lipids, thereby reducing the formation of lipid hydroperoxides.
- Selenium, being a part of glutathione peroxidase, prevents the formation of ROS and destroys (via reduction) hydrogen peroxide and lipid hydroperoxides.
Thus, the occurrence and extent of oxidative damage depends on the antioxidant protective mechanisms of:
- the lipid-soluble vitamin E that is present in the cell membrane, and
- the level of water-soluble Se containing glutathione peroxidase in the aqueous fluid within the cells.
Animals require adequate levels of both Se and vitamin E because of their interrelated functions. The amount of either needed by the animal depends on the availability of the other as illustrated in Figure 2.
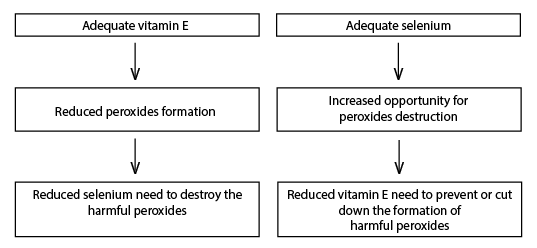 Figure 2.
Figure 2. Interactive need of vitamin E and Se in animal and human diets.
Optimum amounts of both Se and vitamin E are necessary to minimize oxidative damage. Thus, Se and vitamin E should be considered together with regard to both the animal's requirements and the effects and treatment of a deficiency of either.
In Georgia's dairy industry, feeding vitamin E to pregnant and milking cows represents a large cost to producers. There is considerable potential for reducing these costs by focusing more attention on maintaining proper levels of Se in forages and grains.
A number of additional substances also have antioxidant activity and can decrease vitamin E and Se needs when their intake is high, but increase vitamin E and Se needs when their intake is low. These include:
- the sulfur-containing amino acids cystine and methionine,
- vitamin C, and
- the synthetic antioxidant ethoxyquin, which is commonly added to commercially prepared feed.
Certain minerals affect the absorption and metabolism of Se and can alter the dietary Se requirement. These include:
- Calcium: Digestibility and absorption of Se is reduced when cows are fed diets with either high (around 1.4%) or low (around 0.4%) concentrations of calcium, thereby increasing the requirement for dietary Se. Se absorption is maximized when dietary calcium is around 0.8%.
- Sulfur: Increased consumption of sulfur (from sulfate salt) reduces Se absorption, thereby increasing the requirement for dietary Se. Excessive intake of feeds that are high in sulfur such as molasses, beet pulp, plants in the cabbage family, distiller's grains and other corn-distilling co-products like corn gluten feed may aggravate Se deficiency even when the Se level in the diet is adequate. The high sulfur content of Georgia forages may result in higher Se requirement by animals.
- Copper: Adequate copper in the diet helps Se absorption in small ruminants.
Selenium Requirement
The National Research Council recommends the following dietary Se requirements for different classes of animals.
Table 1. Recommended Se concentrations in the diets of selected livestock classes.
| Animal |
Se Requirements (mg/kg diet) |
| Beef Cattle |
0.10
|
| Dairy Cattle |
0.30
|
| Sheep |
0.10–0.20
|
| Growing Pigs |
0.15–0.30
|
| Gestating and Lactating Sows |
0.15
|
| Horses |
0.10
|
| Immature Laying Chickens |
0.10–0.15
|
| Laying Hens |
0.05–0.08
|
| Broiler Chicks |
0.15
|
| Note: Under specific situations, the actual Se requirement may be higher than what has been cited in Table 1. For example, nutrient oxidation for energy needs increases with exercise, which increases the production of ROS, thereby increasing the Se requirement for removing the extra ROS produced. Similarly, a higher desired level of weight gain or milk production may also raise the dietary Se requirement. Even though calculation of dietary Se requirements based on appropriate equations may be higher than what is presented in Table 1, it is important to comply with the regulation of the Food and Drug Administration, which does not allow Se supplementation greater than 0.3 mg/kg in a complete diet. |
Selenium in Georgia Soils and Forages
The Se content of most U.S. soils varies between 0.1 and 2 mg/kg. The maximum reported soil Se content in the U.S. is less than 100 mg/kg. High Se soils (called seleniferous soils) with 2 to 10 mg/kg occur in South Dakota, Montana, Wyoming, Nebraska, Kansas, Colorado and New Mexico.
Soils containing less than 0.5 mg/kg total are classified as Se deficient. According to NRC (1983), Se-deficient regions in the U.S. include New England, New York, New Jersey, Delaware, Pennsylvania, Maryland, West Virginia, Florida, Ohio, Indiana, Illinois, Michigan, Wisconsin, Washington state, Oregon, Montana, Arizona, and coastal regions of Virginia, the Carolinas and Georgia. However, a recent survey revealed that Se-deficient soils may occur throughout Georgia (Figure 3).
Although Se is not an essential element in plant nutrition, the consumption of plants and plant products is the primary route by which animals and humans receive their dietary Se in the absence of any special supplementation. See Table 2 for a general interpretation of selenium and other trace element contents of forages (Mortimer, 1999).
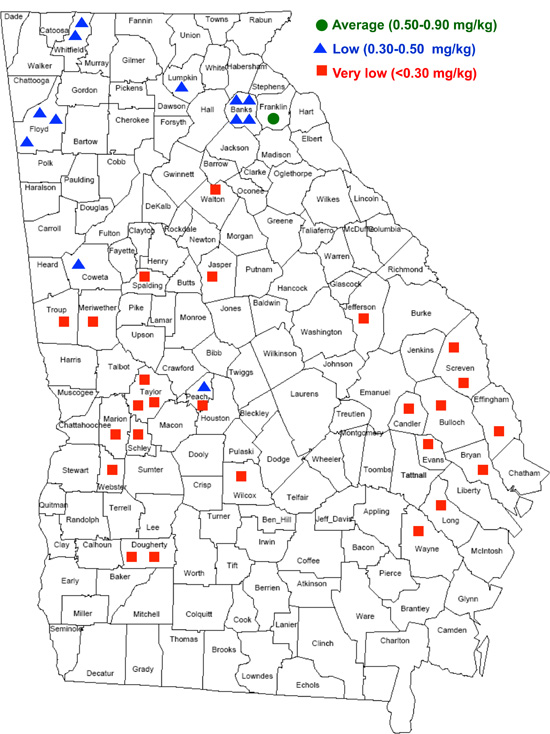 Figure 3.
Figure 3. Soil Se levels in Georgia by county.
Table 2. Classification of trace elements in forages relative to their abilities to meet the dietary requirements of animals [According to Mortimer (1999)]*.
| Element |
Deficient
|
Marginally
Deficient
|
Adequate
|
MTC**
|
| Aluminum (mg/kg) |
-
|
-
|
-
|
1000
|
| Copper (mg/kg) |
< 4
|
4–9.9
|
≥ 10
|
100
|
| Iron (mg/kg) |
< 50
|
-
|
50–200
|
1000
|
| Manganese (mg/kg) |
< 20
|
20–39.9
|
≥ 40
|
1000
|
| Molybdenum (mg/kg) |
-
|
-
|
< 1
|
5
|
| Selenium (mg/kg) |
< 0.100
|
0.100–0.199
|
0.200
|
5
|
| Sulfur (%, Dry Matter Basis) |
< 0.1
|
-
|
0.15–0.2
|
0.4
|
| Zinc (mg/kg) |
< 20
|
20–29.9
|
≥ 30
|
500
|
*This is a general interpretation of forage Se content across all animal types. The requirements change among animals. For example, the requirement for dairy cattle is 0.30 mg/kg.
**Maximum Tolerable Concentration (NRC, 2005). |
In the U.S., most cereal grains for livestock and human consumption are grown in areas with inadequate or moderate Se reserves. Forages and grains (or complete feed) containing less than 0.1 mg/kg Se may result in a Se/vitamin E deficiency disorder. A much higher incidence of this disorder occurs when the Se concentration drops below 0.05 mg/kg.
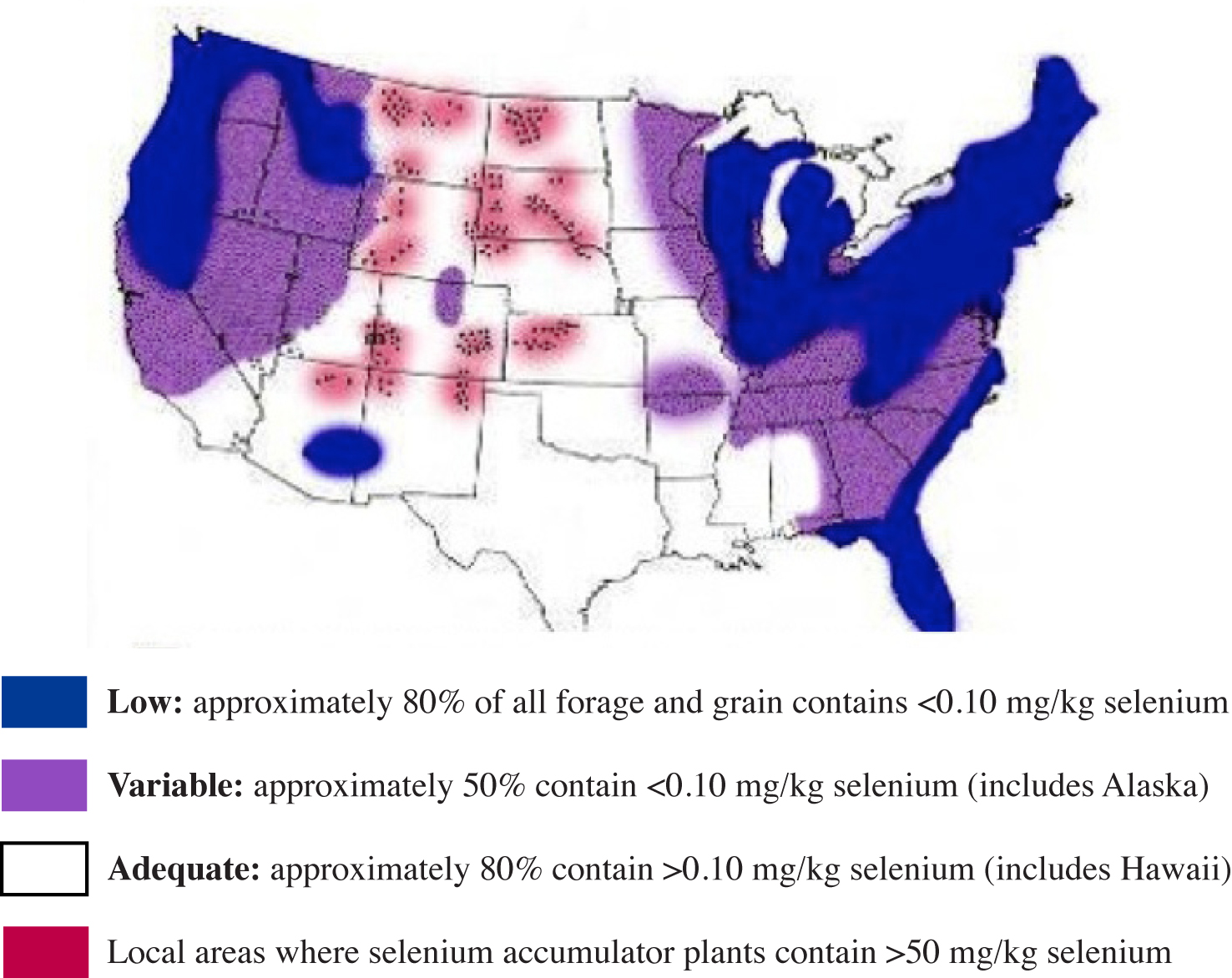 Figure 4.
Figure 4. Regional distribution of forage and grain Se content in the United States (Adapted from NRC, 1983).
The map in Figure 4 was produced by the NRC to show the regional distribution of low, variable and adequate Se levels in forages and grain across the U.S. Areas in the U.S. that produce grains and forages low in Se generally have soils that contain less than 0.5 mg/kg Se.
In NRC's mapping, the forages and grains produced along Georgia's coastline were generally deficient in Se (about 80%). About 50% of the forages and grains produced in the other areas of the state were also Se-deficient (Figure 4).
However, a recently conducted survey of forage Se across the state of Georgia revealed that Se deficiency in Georgia forages is much more severe than what was reported by the NRC's nationwide study (Figure 5).
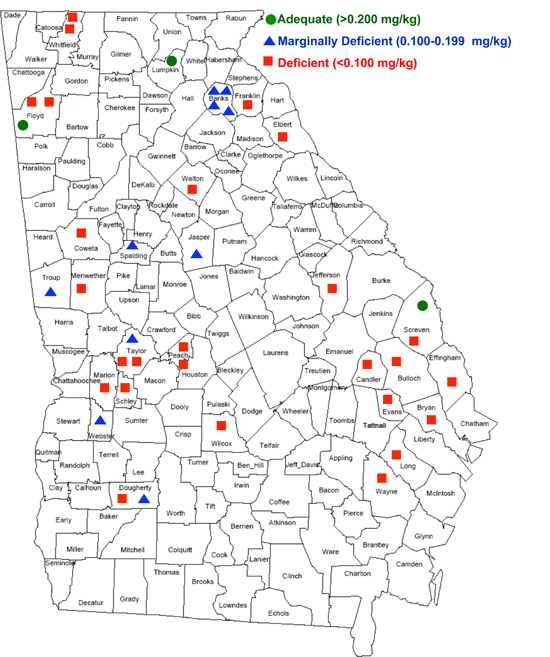 Figure 5.
Figure 5. Forage Se levels by county in Georgia.
Selenium Deficiency
The most severely manifested clinical sign of Se deficiency in animals is called white muscle disease (or nutritional myopathies), which results in degeneration and necrosis in both skeletal and cardiac muscle. This disease may be congenital (present at birth), or it may develop over time. However, a more common consequence of Se deficiency is poor reproductive performance as a result of an increased incidence of early embryonic death and retained placentas. A more detailed listing of Se deficiency signs in ruminants and non-ruminants (i.e., monogastric animals) is given in Figure 6.
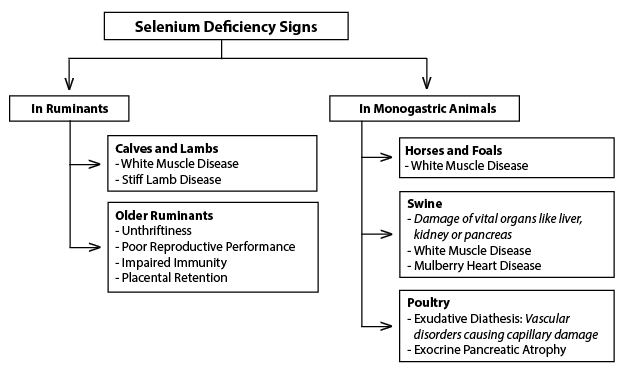 Figure 6.
Figure 6. Selenium deficiency signs and symptoms in various animals.
Clinically, one of the most effective ways to diagnose Se deficiency is by determining the Se concentration in the liver. Liver levels of 0.8-1.0 ppm on a wet weight basis are considered adequate, and levels below 0.2 ppm are considered deficient. Whole blood samples can also be utilized as a general indicator of potential Se deficiencies. Concentrations of 0.05 ppm and below are considered deficient.
In ruminants, sheep are more susceptible than cattle to Se deficiency, and goats are more susceptible than either sheep or cattle. Sudden death within a large, fast-growing population in commercial animal operations is commonly attributed to Se and/or vitamin E deficiency. The phenomenon occurs most frequently in areas of the U.S. where the forages and grains are low in Se, suggesting that Se concentrations in forages and grains are important for preventing deficiency disorders in animals.
In some national-level studies, Georgia was categorized as a state with mild Se deficiency, which often remains hidden without any diagnostic sign. This is certainly damaging to animal performance and can cause substantial loss of revenue in livestock operations; however, the extent of these losses have yet to be fully realized and assessed.
 Figure 7.
Figure 7. Selenium-deficient animals.
http://www.dpi.nsw.gov.au/__data/assets/pdf_file/0016/111355/selenium-deficiency-in-sheep.pdf
http://www.nadis.org.uk/quiz/nerveinjuriescattle.asp (accessed May/24/2011)
Selenium and Sulfur
Sulfur (S) and Se share many of the same physical and chemical properties. These similarities give rise to an important nutritional interrelationship. Upon intake, Se replaces the S of methionine and some other S-containing essential amino acids. The most important and commonly produced of these are selenomethionine (SeMet) and seleno-methyl-selenomethionine. The SeMets are an integral part of the glutathione peroxidase enzyme family—the primary source of antioxidant activity in animal bodies.
Conversely, when levels of S intake become excessive, replacement of S by Se in methionine and formation of SeMet is reduced due to the competitive action of S. The same is true for other S-containing metabolites, resulting in a decrease in Se absorption and an increase in excretion. Thus, the addition of sodium sulfate to the animal diet (total dietary S > 0.33%) may increase the incidence of white muscle disease. Animals grazing on S-fertilized herbage may exhibit lower blood glutathione peroxidase activity than those grazing on unfertilized herbage. As compared to grasses, legumes contain cyanogenic glycosides and higher levels of S, and thus pose a greater potential risk for the development of white muscle disease. The high levels of S in some Georgia forage crops may interfere with proper Se nutrition and should not be ignored when planning Se supplementation in animal feeding.
Selenium Supplementation
Because grain and forage produced in many areas of the U.S., including Georgia, are typically either deficient or marginally deficient in Se, some amount of Se supplementation is required. Current regulations allow the following sources of Se supplementation in livestock operations:
- Inorganic Supplements: Both sodium selenite and sodium selenate are recommended and are more or less equally effective, but because of its lower cost, sodium selenite is more commonly used. The supplements can be provided in several ways:
- Diet: Selenium intake in the diet can be increased by using Se-rich feed ingredients or by providing selenized mineral supplements. The FDA permits manufactured premixes to contain Se of no more than 200 mg/kg and mineral supplements may contain Se not exceeding 11.8 mg/kg. The total Se content of the diet should NOT exceed 0.3 mg/kg or total daily supplement should be no more than 3 mg/head/day. However, this level can be added to the diet regardless of the natural Se content of the feed.
- Direct injection or oral drenching: Sodium selenite solution can be used as an intramuscular or subcutaneous injection or periodic oral drench at 0.1 mg Se per kg live body weight (LBW) for grazing animals that are not provided other concentrate feeds. The suggested application intervals are one to three months for small ruminants, and every three to four months or during the critical production stages for beef and dairy cattle.
- Ruminal placement: A heavy pellet of elemental Se and iron filings may be placed in the reticulum of rumen (forestomach) of ruminant animals. This pellet slowly dispenses Se over an extended period of time (up to one year). It is used in Australia, New Zealand and some European countries; however, this pellet is NOT yet approved for use in the U.S.
Slow-release ruminal glass boluses that contain Se from sodium selenite can be retained in the rumen and release Se over several months.
Another product is an osmotic pump that delivers 3 mg Se per day. The soluble glass boluses, heavy pellets and an osmotic pump are shown in Figure 8.
 Figure 8.
Figure 8. Soluble glass boluses (left), iron-based heavy pellets (back right) and an osmotic pump (front right). Source: Oldfield (1999). Available online at (accessed May/24/2011).
- Organic Supplements: As an alternative to inorganic supplements, FDA-approved Se-enriched yeasts can provide Se in organic form. The organic Se yeast provides a variety of organic Se compounds, but SeMet is the primary component (more than 50% of the total Se) in Se yeasts. Thus, the Se composition of this product closely matches that found in most grains. The SeMet of Se-enriched yeast is readily available and actively absorbed from the intestine. Biotransfer of Se from Se-enriched yeast to milk and fetus (via placental transfer) and retention of Se in meat are substantially higher than from sodium selenite.
Inorganic Versus Organic Selenium Supplementation
In 1974, the FDA approved inorganic Se as a feed supplement and sodium selenite became the most commonly used Se supplement for poultry and other livestock. However, commonly used plant- and animal-based feedstuffs contain Se almost exclusively as organic compounds known as seleno-amino acids. The predominant compound of this class is SeMet. As a supplement, inorganic Se is inferior to organic sources for several reasons. First, a majority of the inorganic Se that is consumed is excreted and therefore unable to build and maintain Se reserves in the body. As a consequence, even if inorganic Se supplementation occurs, it may not provide sufficient Se nutrition in the animal. This is demonstrated in a study that is summarized in Table 3.
Table 3. Blood selenium status of U.S. beef cows and heifers by regions: Effect of inorganic selenium supplementation.
Whole Blood
Selenium Class |
Inorganic Selenium Supplemented Farms
|
Not Supplemented Farms
|
|
West
(19%)
|
Central
(55%)
|
Southeast†
(61%)
|
West
(81%)
|
Central
(45%)
|
Southeast†
(39%)
|
|
% of the total animal examined
|
Severely deficient
(0 to 0.050 mg/L) |
4.4
|
0.0
|
16.7
|
4.9
|
7.9
|
29.1
|
Marginally deficient
(0.051 to 0.080 mg/L) |
8.0
|
3.6
|
23.3
|
4.4
|
9.2
|
32.0
|
Sub-Total (Deficient)
(0 to 0.080 mg/L) |
12.4
|
3.6
|
40.0
|
9.3
|
17.1
|
61.1
|
Adequate
(0.081 to 0.160 mg/L) |
20.3
|
30.9
|
40.9
|
13.1
|
36.5
|
30.8
|
High adequate
(>0.160 mg/L) |
67.3
|
65.5
|
19.1
|
77.6
|
46.4
|
8.1
|
Sub-Total (Adequate)
(>0.080 mg/L) |
87.6
|
96.4
|
60.0
|
90.7
|
82.9
|
38.9
|
| Grand Total |
100
|
100
|
100
|
100
|
100
|
100
|
†Included 53 animals from three Georgia herds.
From: Dargatz and Ross, 1996, J Anim Sci., 74, 2891–2895 |
This study showed that even though inorganic Se supplementation in beef cattle farming is a common practice (in 61% of farms) in the Southeastern U.S. [especially relative to central (55%) and western regions (19%)], Se deficiency was common. Animals produced on farms from these areas demonstrated Se deficiency in blood samples 61% of the time when they were produced without Se supplementation. Even with inorganic Se supplementation programs, 41% of the animals on the Southeastern farms were found to be Se deficient.
Another reason that inorganic Se supplementation strategies are inferior to organic Se supplements is that the selenite is frequently degraded because of interactions with other feed ingredients. A common example is where sodium selenite interacts with vitamin C in the premix and the chemical reaction between them causes reduction of selenite to elemental Se (it often appears as pink particles in the premixes) and oxidation of vitamin C. This reduction of selenite to elemental Se can happen in the premix/feed during storage or even in the digestive tract during digestion and absorption. Some other components in the premix like glucose monohydrate, cornstarch or sucrose can also reduce selenite to elemental Se. Because elemental Se is not absorbed in the digestive tract (hence, it is excreted) and oxidized vitamin C is devoid of biological activity, the nutritional benefit of the Se and vitamin C supplementation is lost if these reactions occur.
Inorganic Se supplementation also can result in potential toxicity via pro-oxidant activity if the supplementation rate is too high. Pro-oxidant activity is the opposite of the desired function (antioxidant activity). Pro-oxidant activity resulting from inorganic Se supplementation may also enhance multiplication of some pathogenic viruses.
Furthermore, the transfer of Se to milk, meat and egg products is inefficient when inorganic Se supplementation is used. This reduces the health benefit to the human diet and is further complicated by pro-oxidant activities of sodium selenite on the shelf-life of milk, meat and eggs.
Selenium Toxicity
Historically, Se toxicity (called selenosis), rather than Se deficiency, was a major concern. The risk of Se toxicity triggered extensive research on Se in animal nutrition (NRC, 2001). As discussed in previous sections, a number of detrimental effects occur if the diet contains less than 0.1 mg/kg Se. However, concentrations exceeding 5 mg/kg Se are harmful and can lead to Se poisoning in livestock (Buck and Osweiler, 1976). The body attempts to rid itself of excess Se through urine, bile and respiration or sequestering it in various tissues, thus making it less available. The portion that overwhelms these excretion and sequestering systems produces toxic effects.
Some plant species, when grown on seleniferous soil, can accumulate Se up to 3,000 mg/kg (NRC, 2000) as compared to 5 mg/kg, which is the maximum tolerable concentration in forages (NRC, 2005). Fortunately, Se accumulator plants usually have an unpleasant garlic-sulfur odor that makes them unpalatable to livestock and grazing animals usually avoid these plants unless pastures are overgrazed or other forages are not available. The risk of grazing Se accumulator plants is low in Georgia because the soils are generally acidic and low in total Se and these Se-accumulating plant species are not found in the state.
Other sources of selenosis risk include excessive administration of inorganic Se supplementation due to improper weighing of Se premixes and administering injectable preparations exceeding the recommended limit. Miller and William (1940) determined the minimum lethal doses of Se from sodium selenite (used for Se supplementation) for horses, cattle and swine as 1.5, 4.5-5.0 and 6-8 mg Se per kg live body weight (LBW).
Responses to toxic levels of Se are characterized as acute (effects manifest within a short time period) or chronic (effects manifest over a long time period). Acute Se poisoning associated with sudden death occurs due to pulmonary congestion and edema and can occur when cattle are fed 10 to 20 mg Se or injected with more than 0.5 mg/kg LBW (NRC 1983; NRC, 2001). In swine, acute selenosis occurs when Se is injected at more than 1.65 mg/kg LBW (Diehl et al., 1975) or fed at more than 20 mg/kg LBW (Mahan and Moxon, 1984). Chronic selenosis has been reported to occur when diets or feedstuffs contain Se at 5 to 20 mg/kg DM for swine (Kim, 1999) or 5 to 40 mg/kg DM for both dairy and beef cattle (NRC, 2000; 2001) over a period of several weeks or months. The recommended dietary Se requirement (0.3 mg/kg) is approximately 16 times less than the lowest dietary level (5 mg/kg) that has been related to chronic selenosis. In chronic excess, Se inhibits cellular enzyme oxidation-reduction reactions, especially those involving S-containing amino acids, methionine and cystine, which affects cell division and growth (Rosenfeld and Beath, 1964). These effects are greatest on hoof and hair, which are the body tissues that contain the highest concentrations of these amino acids, and manifests as clinical signs of abnormal hoof and hair conditions. The common signs of acute and chronic Se toxicosis are depicted below in Figures 9 and 10.
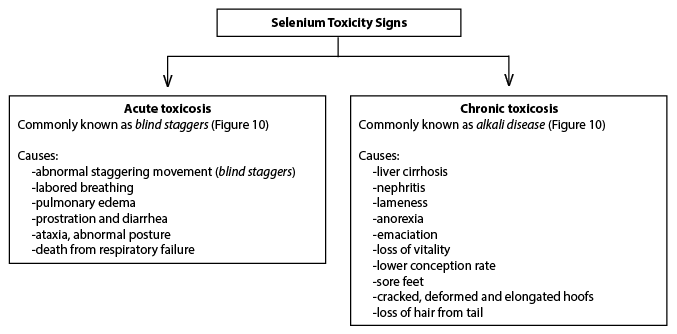 Figure 9.
Figure 9. Common Se toxicity signs in animals.
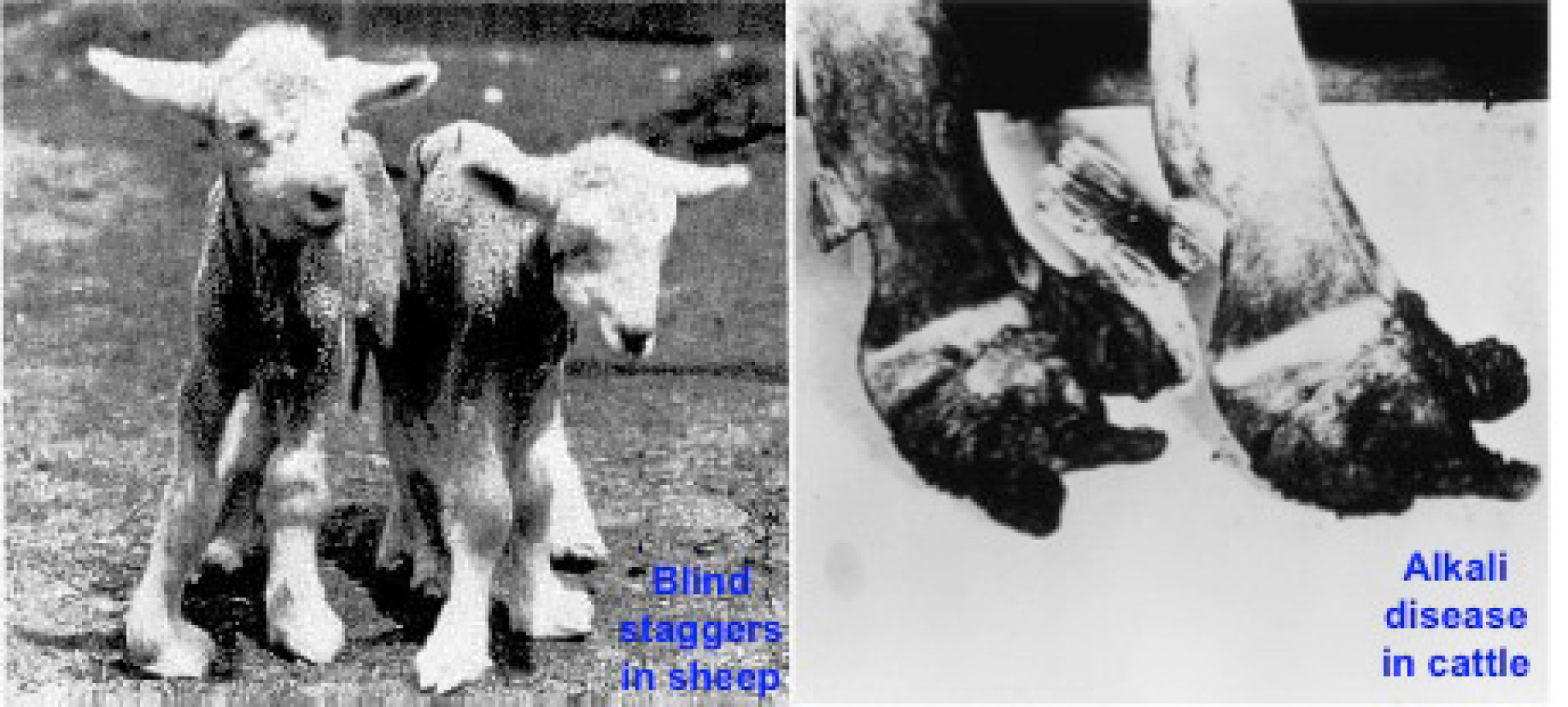 Figure 10.
Figure 10. Blind staggers in sheep caused by acute selenium toxicity (http://www.ansci.cornell.edu/plants/toxicagents/ selenium.html) (left) and Alkali disease in cattle (Oldfield, 2001) (right) caused by chronic Se toxicity. Note the severely damaged hoofs resulting from Se excess.
Summary
- Selenium is a vital micronutrient in animal nutrition that is required in trace amounts. At the same time, it exerts serious toxic consequences, including death, when intake exceeds the tolerance limit.
- Forages and grains used as animal feedstuffs are either deficient or marginally deficient in Se when they are grown on soils with low plant available-Se. Many areas of the U.S., including Georgia, fall in this category.
- In previous nationwide mapping, a large portion of Georgia was shown as marginally deficient in Se, where about 50% of the produced grains and forages are low in Se content (< 0.10 mg/kg). The soils in the Coastal Plain were shown to be clearly Se deficient, producing animal feedstuffs containing low concentrations of Se in 80% of cases.
- A recent study revealed that Se deficiency in Georgia soils and forages is much more severe than previously described. Severe Se deficiency occurs all over the state rather than just in the Coastal Plain.
- Inorganic Se supplementation, as reported for 61% of the beef cattle farms in the Southeast, does not eliminate Se deficiency in the livestock industry. The animals still suffer from varying degrees of Se deficiency that are often not recognized.
- Se deficiency in Georgia's livestock industry is a hidden problem that reduces animal performance and profitability, and merits more attention, assessment and management.
- The geographical coincidence of Se deficiency in animals and low Se contents of forages and grains used as feed in the U.S. suggests that adequate Se concentrations in feedstuffs are important in preventing Se-related health problems in animals.
References
Buck W. B., & Osweiler, G. D. (1976). Selenium. In W. B. Buck, G. D. Osweiler, & G. A. van Gelder (Eds.), Clinical and diagnostic veterinary toxicology (2nd ed.). Kendall Hunt Publishing.
Diehl, J. S., Mahan, D. C., & Moxon, A. L. (1975). Effects of single intramuscular injections of selenium at various levels to young swine. J. Anim. Sci., 40, 844–850. https://doi.org/10.2527/jas1975.405844x
Kim, Y. Y. (1999). Selenium metabolism and toxicity of inorganic and organic selenium sources and levels on growth, reproduction, and other mineral nutrients in swine [Doctoral thesis]. Ohio State University.
Mahan, D. C. & Moxon, A. L. (1984). Effect of inorganic selenium supplementation on selenosis in postweaning swine. J. Anim. Sci., 58, 1216–1221. https://doi.org/10.2527/jas1984.5851216x
Miller, W. T., & Williams, K. T. (1940). Minimum lethal dose of selenium as sodium selenite for horses, mules, cattle and swine. J. Agric. Res., 60, 163–173.
Mortimer R. G., Dargatz, D. A. & Corah, L.R. (1999, April). Forage analyses from cow-calf herds in 23 states (Report No. N303.499). USDA–APHIS:VS, Centers for Epidemiology and Animal Health.
National Research Council (NRC) Subcommittee on Selenium. (1983). Selenium in nutrition. National Academies Press. https://doi.org/10.17226/40
NRC Subcommittee on Beef Cattle Nutrition. (2000). Nutrient requirements of beef cattle (7th revised ed.). National Academies Press. https://doi.org/10.17226/9791
NRC Subcommittee on Dairy Cattle Nutrition. (2001). Nutrient requirements of dairy cattle (7th revised ed.). National Academies Press. https://doi.org/10.17226/9825
NRC Committee on Minerals and Toxic Substances in Diets and Water for Animals. (2005). Mineral tolerance of animals (2nd revised ed.). National Academies Press. https://doi.org/10.17226/11309
Oldfield, J. E. (2001). A brief history of selenium research: From alkali disease to prostate cancer (from poison to prevention). J. Anim. Sci., 11(1), 1–4. https://jeffreydachmd.com/wp-content/uploads/2013/04/Selenium-From-alkali-disease-to-prostate-cancer-from-poison-to-prevention-Oldfield-2002.pdf
Rosenfeld, I., & Beath, O. A. (1964). Selenium: Geobotany, biochemistry, toxicity, and nutrition. Academic Press. https://doi.org/10.1016/B978-1-4832-2800-6.50005-5
We gratefully acknowledge the contributions provided by these additional original publication authors: Leticia Sonon, Jake Mowrer, and Dennis Hancock, former ½ûÂþÌìÌà Extension faculty; and Nicholas Hill and David Kissel, retired ½ûÂþÌìÌà Extension faculty.
Status and Revision History
Published on Aug 12, 2011
Published with Full Review on Aug 03, 2014
Published with Full Review on Aug 03, 2023