Revised by Uttam Saha, Leticia Sonon, Mark Risse1 and David Kissel
Agricultural and Environmental Services Laboratories
1Marine Extension and Georgia Sea Grant
Originally written by Anthony Tyson and Kerry Harrison, Extension Engineers
Introduction

 Figure 1:
Figure 1: An abundant supply of clean, safe drinking water is essential for human and animal health.
(Sources: Top photo: authors; Bottom photo: www.photobucket.com).
An abundant supply of clean, safe drinking water is essential for human and animal health. Water from municipal or public water systems is treated and monitored to ensure that it is safe for human consumption. Many Georgia residents, especially in rural areas, rely on private water systems for human and livestock consumption. Most private water systems are supplied by wells.
Water from wells in Georgia is generally safe for consumption without treatment. Some waters, however, may contain disease-causing organisms that make them unsafe to drink. Well waters may also contain large amounts of minerals, making them too “hard” for uses such as laundering, bathing or cooking. Some contaminants may cause human health hazards and others can stain clothing and fixtures, cause objectionable tastes and odors, or corrode pipes and other system components.
Surface water sources, such as springs and cisterns, are seldom used for drinking water. They are almost always contaminated with pathogenic microorganisms; therefore, surface water should always be treated before being consumed.
The quality of drinking water from private sources is the responsibility of the homeowner. State laws do not require testing of private domestic water supplies, and regulatory agencies do not regularly monitor the quality of water from private supplies. Therefore, the only way homeowners can be certain that their water is safe to drink is to have it tested periodically.
Drinking Water Standards
The EPA standards for drinking water fall into two categories: Primary Standards and Secondary Standards. The current standards for some selected primary and secondary contaminants are listed below in Tables 1 and 2. For further details about EPA's drinking water standards, visit
Primary Drinking Water Standards
Primary Standards are based on health considerations and are enforced by the EPA in public water systems. They protect you from three classes of toxic pollutants: microbial pathogens, radioactive elements and organic/inorganic chemicals. Many of these contaminants occur naturally in trace amounts in ground or surface water. Primary Standards set a limit, called the Maximum Contaminant Level (MCL), which is the highest allowable concentration of a contaminant in drinking water supplied by public water systems. The MCL is an enforceable health goal based on calculated risk due to exposure for a period of time. For example, EPA calculates health risk for arsenic based on assumption that an individual would drink from a singular water source for 70 years. Besides MCL, Primary Standards set another limit, called Maximum Contaminant Level Goal (MCLG), the level of a contaminant in drinking water below which there is no known or expected risk to health. MCLGs allow for a greater margin of safety than MCLs, but these are non-enforceable public health goals. Both MCL and MCLG are usually expressed in milligrams per liter (mg/L) or parts per million (ppm), which are numerically equivalent. Table 1 lists the current primary drinking water standards, including organic/inorganic chemicals, radioactive elements and microbial pathogens.
| Table 1. Current Primary Standards for Drinking Water. |
| Contaminant |
Maximum Contaminant
Level (mg/L) |
Maximum Contaminant
Level Goal (mg/L) |
| Inorganics |
Arsenic
Barium
Bromate
Copper
Cadmium
Chromium (total)
Fluoride
Lead
Mercury (inorganic)
Nitrate (as N)
Nitrate (as NO3)
Selenium
Uranium |
0.010
2.00
0.010
1.300
0.005
0.100
4.000
0.015
0.002
10.00
45.00
0.050
0.030 |
0
2.00
0
1.300
0.005
0.100
4.000
0
0.002
10.00
45.00
0.050
0 |
| Organics |
Endrin
Lindane
Methoxychlor
Toxaphene
2, 4-D
2, 4, 5-TP (Silvex)
Total Trihalomethanes |
0.002
0.0002
0.040
0.003
0.07
0.050
0.080 |
0.002
0.0002
0.040
0
0.07
0.050
n/aa |
| Volatile Organic Chemicals |
Benzene
Carbon Tetrachloride
Para-dichlorobenzene
1, 2-dichloroethane
1, 1-dichloroethylene
1, 1, 1-trichloroethane
Trichloroethylene
Vinyl Chloride |
0.005
0.005
0.075
0.005
0.007
0.200
0.005
0.002 |
0.005
0.005
0.075
0.005
0.007
0.200
0.005
0.002 |
| Radionuclides |
Radium 226 and 228 (combined)
Gross Beta Particles
Gross Alpha Particles |
5 pCi/Lb
50 PCi/L
15 pCi/L |
5 pCi/Lb
50 PCi/L
15 pCi/L |
| Microbiological |
Total Coliform
Fecal coliform and E. coli
Turbidityd |
0c
0
1-5 turbidity units
|
0c
0
N/Ae |
a Although there is no collective MCLG for this contaminant group, there are individual MCLGs for some of the individual contaminants: bromodichloromethane (zero); bromoform (zero); dibromochloromethane (0.06 mg/L): chloroform (0.07mg/L).
b Concentrations of radioactive elements are measured in picocuries per liter (pCi/L), a measure of radioactivity.
c ‘Colony Forming Units (CFU)’ or ‘Most Probable Number (MPN)’ in a 100 milliliter sample of water.
d Turbidity is a measure of the cloudiness of water. It is used to indicate water quality (e.g., whether disease-causing organisms are present) and filtration effectiveness. Higher turbidity levels are often associated with higher levels of disease-causing microorganisms such as viruses, parasites and some bacteria. These organisms can cause symptoms such as nausea, cramps, diarrhea and associated headaches.
e Not available. |
Secondary Drinking Water Standards
Secondary Drinking Water Standards regulate constituents that cause offensive taste, odor, color, corrosivity, foaming and staining. The concentration limit is called the Secondary Maximum Contaminant Level (SMCL). Secondary Standards are not enforceable. Public water systems are not required to test for or remove secondary contaminants. Secondary Standards are guidelines for water treatment plant operators and state governments attempting to provide communities with the best quality water possible. See Table 2 for Secondary Drinking Water Standards, including inorganic chemicals and physical problems.
| Table 2. Current Secondary Standards for Drinking Water. |
| Constituents |
Secondary Maximum
Contaminant Level |
| Inorganics (mg/L) |
Chloride
Copper
Fluoride
Foaming Agents
Iron
Manganese
Silver
Sulfate
Total Dissolved Solids (TDS)
Zinc |
250
1.00
2.00
0.5
0.3
0.05
0.100
250
500
5 |
| Physical Problems |
Color
Corrosivity
Odor
pH |
15 color units
noncorrosive
3 TON (threshold odor number)
Not less than 6.5 and not greater
than 8.5 on pH scale |
Water Testing
To ensure that water is safe for human consumption and livestock use, water supplies should be tested and checked to ensure they meet the acceptable levels for bacterial and chemical contents.
The local health department in most counties can conduct a microbiological test. ½ûÂþÌìÌà Cooperative Extension can conduct both microbiological and chemical or mineral analysis. Many private laboratories also offer these tests. To test your water, call your local county Extension office (1-800-ASK½ûÂþÌìÌÃ1) or check with your municipal water supplier or county health department to find a certified private laboratory near you. A list of water testing laboratories certified by Georgia Department of Natural Resources is also available at .
The testing procedure is not the same for all contaminants. Call your local Extension office, health department or private lab for appropriate bottle(s) and instructions on sample collection and submission. After sending a water sample to a laboratory, the laboratory will then return a report indicating what is found in your water, including those contaminants that exceed standard levels (MCLs or SMCLs). Treatment options are also recommended when necessary.
Specific questions about water quality can often be answered with the right test. Unfortunately, no single water test can provide you with information on all possible contaminants. Public water supply systems typically spend few thousand dollars to analyze for the EPA-required suite of all primary and secondary contaminants that may be found in drinking water. Such a comprehensive testing is expensive, impractical and may not be necessary for a domestic well. Instead, tests for some common constituents are recommended as discussed below.
Mineral Analysis
A mineral analysis checks for the inorganic constituents found in water. A typical mineral analysis will give the content in parts per million (milligrams per liter) of mineral elements such as calcium, magnesium, manganese, iron, copper and zinc. It will also determine the acidity or pH of the water and the hardness, expressed in parts per million or grains per gallon. It may also give the concentration of nitrate, sulfates and other chemical compounds.
Large amounts of minerals and other impurities may pose a health hazard. For instance, nitrate contamination can cause health problems for infants and ruminant animals, sulfates can have a laxative effect in humans, and arsenic may cause cancer if consumed over a long period of time. Minerals at high concentrations can also affect the appearance and use of the water. Hard water is due to high levels of calcium and magnesium. Iron may leave red stains on plumbing fixtures, equipment and laundry. Suspended silt makes water look muddy or cloudy and dissolved gases may give water a bad taste and/or odor.
Microbiological Tests
A microbiological test tells you if your water is at risk for contamination by disease-causing microorganisms (pathogens). However, testing drinking water for all possible pathogens is complex, time consuming and expensive. Instead, water is commonly tested for indicator microorganisms such as total coliform and E. coli bacteria because if they are present in water, the condition of the well and its surrounding environment may support the presence of other disease-causing microorganisms. Thus, a positive water test result for total coliform only or both total coliform and E. coli indicate the possible existence of various disease-causing microorganisms.
Coliform is a group name that includes many bacteria. Most coliform bacteria do not cause disease and they are abundant in soils, waters, vegetation, etc. However, their presence in drinking water indicates that disease-causing organisms could be contaminating the water system. E. coli is a special type of coliform bacteria that most likely originates from a fecal source. Most E. coli bacteria are also harmless and are found in great quantities in the intestines of people and warm-blooded animals. However, some E. coli strains (for example E. coli O157:H7) can cause illness. The presence of E. coli in a drinking water sample almost always indicates recent fecal contamination by sewage or manure, meaning there is a greater risk that pathogens are present. For further information about coliform bacteria, refer to University of Georgia Cooperative Extension Circular 858-7, Your Household Water Quality: Coliform Bacteria in Your Water.
Pesticide and Other Organic Chemical Tests
There are many man-made chemicals that can potentially contaminate a water supply if they are not disposed of properly. These chemicals may impair water quality and cause a health hazard. Examples of these chemicals include petroleum products, industrial chemicals and agricultural pesticides.
Chemicals that are not part of a laboratory's routine suite of analysis are not typically analyzed unless a particular type of chemical is suspected to be in the water. It can be very expensive to test for the presence of many unknown chemical contaminants; however, if a particular chemical is suspected, a test can usually be performed at a moderate cost.
½ûÂþÌìÌÃ's Recommended Guidelines for Testing Drinking Waters
½ûÂþÌìÌà Water Testing Laboratory recommends the following guidelines for testing drinking waters.
Household Drinking Well Water
- Expanded Water Test (W2): Designed to address common well water problems in Georgia such as corrosion and scaling, high levels of iron and manganese, saltwater intrusion and nitrate from various sources. It is also useful for water treatment design. This test includes:
- Basic Water Test (W1): pH and Hardness, Phosphorus (P), Potassium (K), Calcium (Ca), Aluminum (Al), Boron (B), Chromium (Cr), Copper (Cu), Cadmium (Cd), Iron (Fe), Magnesium (Mg), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), Silica (Si), Sodium (Na), Zinc (Zn)
- Anions (W3): Chloride (Cl), Fluoride (F), Nitrate (NO3), Phosphate (PO4), and Sulfate (SO4)
- Soluble Salts (W11): Electrical Conductivity/ Specific Conductance
- Alkalinity (W18)
This test package should be done at least once initially before using a less inclusive test package such as W1. It should be repeated once every three years.
- Basic Water Test (W1): Recommended annually only after an initial W2 has been conducted.
- Total Coliform/E. coli (W35): Annually
- Lead (W9): Initial + semi-annually. If your house was built before 1985, pipes could contain lead solder, which could leach into your drinking water. However, if your home is piped with PVC (as found in newer construction) and if the initial testing does not show any concern about lead there is no need for subsequent testing.
- Hydrogen Sulfide: If your water smells like rotten eggs.
Small Drinking Water Providers (Small Distribution Systems)
- W33 and W35: W33 is a test designed for Georgia Certification for Drinking Water Providers (Small Distribution Systems). This includes:
- Basic Water Test (W1) and anions (W3)
- Total Nitrate (NO3-N) + Nitrite (NO2-N) Basic Water Test (W1)
- Nitrite-Nitrogen
- Soluble Salts
- Alkalinity
- Total Dissolved Solids
- Color
- Turbidity
Collecting a Water Sample
Before collecting water for testing, contact the laboratory or agency that will perform the test. They should be able to provide you with a set of instructions and an appropriate container(s) for sample collection.
Make sure you collect the sample from a faucet that has had the aerator removed. Some tests require a first draw water sample, whereas others require flushing of the faucet and the pipes by running water for several minutes before sampling. Collect the sample, making sure not to touch the inside of the bottle or let the water run over your hand before entering the bottle. Cap the bottle immediately and deliver it to the laboratory as quickly as possible. Note that the samples for some water tests are required to be analyzed within a certain specified period after collection, called holding time. Your laboratory should provide you the information about holding time requirements (if any).
To ensure that a well water supply is safe, it should be tested for bacteria and nitrates at least once a year. A complete mineral analysis (equivalent to W2 of the ½ûÂþÌìÌà water testing laboratory) should be performed at least every three years.
A simplified sampling protocol for the major drinking water tests offered by the University of Georgia's Water Laboratory is given below (Table 3).
| Table 3. A simplified sample collection protocol for some major drinking water tests available at ½ûÂþÌìÌÃ's water testing laboratory. |
| Tests |
Bottle: Size and Type |
Sampling |
W1: Basic Water Test
(pH, minerals and hardness) |
125 mL Plastic (4 oz.) |
Collection Spot:
A kitchen faucet or a faucet used most often for drinking and cooking.
Protocol:
1. A first draw water sample will be collected either early morning or evening upon returning home to ensure that the necessary 6-12 h stagnant water conditions exist.
2. Place a clean sample container below the faucet and gently open the cold water tap. Completely fill all sample bottles, being careful not to contaminate the sample. To prevent contamination, do not touch the inside of the bottle or lid.
|
W2: Expanded
Water Test
(Useful for Water Treatment Design:
W1 + Anions + Soluble Salts + Alkalinity) |
500 mL Plastic (16 oz.) |
| W9: Lead |
125 mL Plastic |
W33: Detailed
Water Test
(Required for Georgia – Certification for Drinking Water Providers: W2 + Nitrite-N + Combined Nitrate & Nitrite-N + Total Dissolved Solids + Color + Turbidity) |
1 quart Plastic (+ 125 mL by overnight shipment if turbidity is critical) *
|
* In W33, turbidity may be measured from a 1-quart sample if turbidity is NOT critical. |
| Arsenic and Uranium |
125 mL Plastic (4 oz.) |
Collection Spot:
A kitchen faucet or a faucet used most often for drinking and cooking.
Protocol:
1. Turn on the cold water to its full capacity and let it run to waste for 2 to 3 minutes to flush the water out of the pipes.
2. Turn the faucet down to a pencil size stream of water and fill a 125 mL plastic sample bottle, being careful not to contaminate the sample. To prevent contamination, do not touch the inside of the bottle or lid.
|
| W35-W40 (Microbiological Tests) |
100 ml special plastic bottle with essential dechlorinating agent (white powder) available at Feed and Environmental Water Laboratory and ½ûÂþÌìÌà county Extension offices. |
Collection Spot:
A kitchen faucet or a faucet used most often for drinking.
Protocol:
1. Select an inside faucet that is clean and not leaking.
2. Remove any faucet attachments such as filters, aerators, screens, splashguards or water-saver valves.
3. Sanitize the faucet inside and out by dipping the faucet neck into undiluted chlorine bleach (do not use color-safe bleach).
4. Open tap fully and flush the faucet and pipes by running water for 3 minutes. If sampling from a faucet that mixes hot and cold water, run hot water for 3 minutes and turn it off, then run cold water for 3 minutes. Do not turn off the cold water, but reduce the flow to avoid splashing.
5. Uncap the sample bottle without touching the inside of the cap or bottle, fill the bottle above the 100 mL line (but not completely full) and recap tightly. Fill the bottle only once; do not rinse.
6. Submit the sample to the laboratory in person or ship it by overnight delivery option because the sample must be received by the laboratory within 24 hours of collection.
|
Common Water Quality Problems and Solutions
The most common water quality problems encountered by private well owners in Georgia include contamination by bacteria, abnormal taste, odor, corrosiveness, hardness, and high levels of iron and manganese. In some situations, well waters contain some inorganic contaminants like nitrate, lead, copper, arsenic and uranium at levels higher than EPA's maximum contaminant levels (MCL). Problems of organic contaminants (for example, pesticides and petroleum hydrocarbons) are not as common as the inorganic contaminants.
In some water quality impairment situations (for example, copper and lead dissolution from the plumbing system), managing the sources of the problem is more cost effective in the long run than installing and maintaining a water treatment system. It is important to know that one single type of treatment cannot address all types of water quality problems. You should purchase the appropriate type of treatment system that will effectively solve your unique water quality issue. The ½ûÂþÌìÌà water testing laboratory report contains an unbiased interpretation and directions for treatment options if an MCL is exceeded. Table 4 can be a useful guide for identifying water quality problems with your well, sources of contaminants causing the problems and possible solutions.
| Table 4. Common water quality problems, their sources and possible solutions.1 |
| Symptom |
Probable Cause |
Possible Health Effects |
Standard/ Guideline |
Suggested Remedy |
|
Sudsy lather difficult to maintain in wash basin.
Greasy-grimy ring in bathtub.
White, scaly deposits in pipes or appliances or on glassware.
|
Hard water due to calcium and magnesium compounds dissolved from rocks and minerals in the earth. |
No health hazard.
Generally contributes a small amount to total calcium and magnesium dietary needs.
|
Guidelines
Hardness as grains per gallon:
0 to 0.99 = soft
0.99 to 3.5 = slightly hard
3.5 to 7.0 = moderately
hard
7.0 to 10.5 = hard
Over 10.5 = very hard |
Install a water softener to remove calcium and magnesium compounds. |
|
Water appears clear when first drawn from tap, but if allowed to sit, fluffy brown or reddish-brown particles begin to form and settle to the bottom or water turns reddish-brown during cooking/ heating.
Reddish-brown stain in sinks, toilets and bathtubs.
Water has metallic taste.
|
Dissolved iron in ground water from natural sources gets oxidized by air and forms an insoluble rusty iron oxide.
Indicates more than 0.3 mg/L dissolved iron.
|
No known health risk. |
Secondary MCL
Iron: 0.3 ppm |
Shock chlorinate the entire well and plumbing systems (for a step-by-step shock chlorination procedure, see the footnote 2 of this Table). If problem returns, use:
Polyphosphate feeder for low levels of dissolved iron and manganese at combined concentrations up to 3 mg/l.
High capacity water softener recommended by the manufacturer for low to moderate levels of dissolved iron and manganese at combined concentration up to 5 mg/l.
Oxidizing filter (manganese greensand or zeolite or manganese oxide) for moderate levels of dissolved iron and manganese at combined concentrations up to 15 mg/l.
Aeration (pressure type) followed by mechanical filtration for high levels of dissolved iron and manganese at combined concentrations up to 25 mg/l.
Chemical oxidation with potassium permanganate or chlorination or ozonation followed by mechanical (sand) filtration for any level of dissolved or oxidized iron and manganese greater than 10 mg/l.
|
|
Reddish-brown or black slime on walls of toilet flush tank.
Slimy materials suspended in clear water.
Reduced pumping capacity.
|
Iron-eating bacteria live in pipes and produce slimy material that hardens into scale. |
No known health risk. |
|
Disinfect the entire well and plumbing systems with shock chlorination; if condition persists, install a continuous chlorinator sand filter. |
|
Iron pipe rusts.
Water dripping from faucet has rusty color.
Blue-green stains on sink.
|
Low pH or high concentration of carbon dioxide and/or low ionic strength water.
Copper tubing corrodes due to low ionic strength and/or low pH water.
|
Too much copper can cause permanent kidney and liver damage in infants. Adults with “Wilson’s Disease” are seriously affected. |
A good guide for well owners is to maintain a pH level of 6.5-8.5.
Primary MCL for copper is 1.3 ppm.
Secondary MCL for copper is 1.0 ppm.
|
Use a calcium carbonate neutralizing filter plus water softener OR Feed soda ash solution into water system with automatic chlorinator.
If possible, consider replacing copper pipes with PVC pipes in the plumbing system.
|
|
Rust particles or black specks suspended in clear water.
Brownish-blackish stains on fixtures and laundry.
Diminished flavor and color of water and cooked food.
|
Dissolved manganese in water gets oxidized and forms precipitates.
Natural deposits of manganese in underground rock formation dissolved into ground water, often combines organics.
Usually occur simultaneously with dissolved iron.
|
No known health hazard. |
Secondary MCL
Manganese: 0.05 ppm |
Shock chlorinate the entire well system (see footnote 2). If problem returns:
For low level of manganese, use ion exchange water softener.
For higher levels use: Oxidizing filter; continuous chlorination or ozonation; or aeration followed by sand filtration.
|
| Water feels greasy and black stains appear in sink, often with rotten egg odor. |
Iron and/or manganese sulfides due to the presence of sulfate-reducing bacteria in water supply. |
No known health hazard. |
Secondary MCL
Iron: 0.3 ppm
Sulfate: 250 ppm
|
Shock chlorination (see footnote 2) followed by discharge of large amount of water until chlorine and rotten egg odor dissipates. If the problem returns:
Install a continuous chlorination system followed by activated carbon filtration.
Install an iron and sulfur water conditioner.
|
| Rotten egg odor from both cold and hot water taps. |
Hydrogen sulfide, sulfur, or sulfate-reducing bacteria in ground water. |
Not usually a health risk at concentrations present
in household water; however, hydrogen sulfide gas is flammable and poisonous if released at high concentration. |
Secondary MCL
Sulfate: 250 ppm |
Shock chlorination (see footnote 2) followed by discharge of large amount of water until chlorine and rotten egg odor dissipates. If the problem returns:
Install a continuous chlorination system followed by activated carbon filtration.
Install an iron and sulfur water conditioner.
|
| Rotten-egg odor in hot water only. |
Chemical reaction of magnesium rod in hot water heater in presence of soft water. |
No known health risk. |
|
Replace magnesium rod from heater with an acceptable alternative such as an aluminum rod. |
| Objectionable taste or odor other than hydrogen sulfide (e.g., a musty, earthy or woody smell). |
Decaying organic matter.
Pollution from surface drainage.
Insufficient chlorine being used to disinfect water.
|
No known health risk in case of decaying organic matter. |
Secondary Standard
Odor: 3 TON (threshold odor number)
|
Install an activated carbon filter OR
Install an automatic chlorinator followed by an activated carbon filter.
|
| Chlorine smell. |
Excessive chlorination. |
Chlorine in water is not poisonous to humans or animals.
High concentrations can cause imitation to tongue and make water taste odd.
|
Maximum residual
chlorine allowed: 4.0 ppm |
Install an activated carbon filter. |
| Detergent odor or foaming water. |
Septic tank leakage into water supply. |
Gastrointestinal illnesses (diarrhea, vomiting, cramps). |
|
Eliminate source and shock chlorinate well (see footnote 2). |
| Methane gas odor. |
Naturally decaying organic substances found in shallow wells near swamps.
Houses built above/near old landfills or aquifers overlying oil fields.
|
Gas is toxic to breathe and explosive. |
If concentrations are above 28 mg/L, the U.S. Department of the Interior, Office of Surface Mining suggests that you take immediate action to reduce this concentration. Concentrations of 10 mg/L or less are considered safe. |
A well vent can remove methane from some wells. Contact a certified well contractor in your area to see if a well vent can be installed on your well. Aeration can also be used to remove methane.
Install a residential/ commercial deaeration system and re-pump.
|
| Gasoline or oil smell. |
Fuel tank or underground storage tank leaking into water supply.
Discharge from factories or landfills.
Run-off from agriculture.
|
Varies depending on the contaminant, possibly: Anemia; increased risk of cancer; or liver and kidney problems.
|
Primary Standard
Benzene <0.005ppm
Ethyl benzene <0.7 ppm
Toluene <1.0 ppm
Xylenes <10 ppm
Guideline
MTBE <0.20 ppm
|
Eliminate the source.
Install an activated carbon filtration system.
|
| Sharp chemical odor in water (may also be odorless). |
Leaching of pesticides into groundwater. |
Anemia or other blood disorders.
Nervous system or reproductive disorders.
Increased risk of cancer or stomach, liver, kidney problems, etc.
|
EPA has specific standards for many pesticides. |
Activated carbon filter OR
Reverse osmosis.
|
| Turbid, cloudy or dirty water with suspended particles that settle out in water. |
Suspended particles of silt, clay and colloidal matter. |
Harmful contaminants
may be attached to soil/clay particles. |
|
Install:
A new well screen AND
A cartridge-type sediment filter OR
An automatic sand filter.
|
| Water unsafe or not potable due to coliform bacteria. |
Contamination due to sewage, manure or surface runoff. |
Health risk due to water-borne disease-causing microorganisms. |
Primary MCL
Coliform bacteria: 0 |
Shock chlorinate the well and plumbing systems (see footnote 2). If the condition persists, install:
Continuous chlorinator
Ultraviolet disinfection equipment OR
Distillation system.
|
1 Adapted from “Drinking Water Treatment: An Overview,” EC703, Institute of Agriculture and Natural Resources at the University of Nebraska-Lincoln. Available online at http://extensionpublications.unl.edu/assets/pdf/ec703.pdf.
2 For a step-by-step procedure for shock chlorination, refer to the University of Georgia publication" Disinfecting Your Well Water: Shock Chlorination," available at your local Extension office and online at: http://extension.uga.edu/publications/detail.cfm?number=C858-4 |
Water Treatment
Many different types of water treatment systems are available in the current market. In general, water treatment systems are based on one or a combination of the following basic treatment methods:
- Filtration such as mechanical filters, activated carbon filters, oxidizing filters, neutralizing filters, microfiltration, etc.
- Ion exchange (water softeners)
- Chemical oxidation
- Disinfection methods such as chlorination, ultraviolet light, ozonation, etc.
- Reverse osmosis
- Distillation
Note that one particular type of treatment system cannot take care of all kinds of water quality problems and a combination of methods may often be needed.
Depending on the nature and extent of contamination, most of the above treatment methods offer two major types of water treatment devices:
- Point of Entry (POE)
- Point of Use (POU)
Point of Entry Water Treatment Systems
Point of Entry (POE), or whole house treatment systems, treat all water entering the home. They are more expensive and are for treating a larger volume of water. They are useful when the water has problems that affect all areas of the home. The most common example is a POE water softening ion exchange system that removes calcium and magnesium ions (and some other ions) from hard water. Even though hard water is not unhealthy to drink, it can cause scale buildup in pipes and on fixtures, interfere with the effectiveness of soap and shorten the life of appliances like dish washers and hot water heaters. Other POE water treatment systems are also designed to remove iron and manganese, adjust pH levels and add chlorine or other disinfectants. The POE devices typically treat about 100-300 gallons per day, depending on family size.
Point of Use Water Treatment Systems
Point of Use (POU) systems treat water at the point where it is used. These are the systems that are installed at a specific location, frequently at the kitchen sink, to treat only the water that is used for drinking, cooking, etc. However, if other ports like the one on a refrigerator and/or a bathroom sink are also used for drinking, POU treatment systems should be installed at those locations as well to ensure total safety of drinking water. POU devices typically treat only a few gallons of water per day, and generally only water that will be directly consumed or used for cooking needs to be treated. Such a system might be used for contaminants like arsenic, cadmium, chromium, fluoride, uranium, nitrate or radium, some organic chemicals and sodium. Reverse osmosis, distillation and activated carbon units are generally POU devices because their main purpose is to provide few gallons of clean water per day for drinking and cooking only. It is cost prohibitive (and generally not necessary) to install them as POE devices to treat all water entering the home.
Some Common Water Treatment Techniques
Distillation
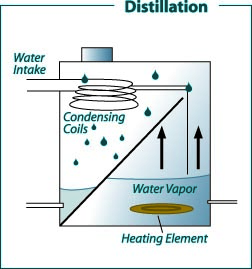 Figure 3:
Figure 3: Domestic distillation water treatment system.
(Recreated from: FilterWater.com http://www.filterwater. com/asp/cs/water-purification-technologies.asp)
Distillation is a treatment method that normally removes more than 99.9 percent of the dissolved minerals in water. Tap water in a tank (often made of stainless steel) is heated to boiling. Bacteria are killed during boiling. The steam produced enters condensing coils, where it is cooled and condensed back to water. The distilled water goes into a storage container or is piped to a special faucet. Storage containers can be glass, metal or plastic. Dissolved minerals and other dissolved and suspended substances are left behind when the steam enters the condensing unit. Thus, distilled water is considered relatively pure. Unevaporated contaminants are left behind and periodically flushed to the septic or sewer system. These units are very effective in removing almost all common water contaminants. The only exception is volatile organic chemicals, which have a boiling point less than or near that of water (for example, some pesticides and volatile solvents) and are vaporized and carried into the condensation chamber and condensed with the distilled water.
Some distillation units have a volatile gas vent that releases these vaporized contaminants to the atmosphere. Filtering the distilled water through an activated carbon filter is another way to remove most of these organic chemicals. Except for small portable units, most home distillation stills are connected to the household plumbing. This maintains a constant supply of feed water, which permits continuous operation and production. A typical household unit will produce between 4 and 12 gallons of treated water per day, depending on the size of the heating element. The known disadvantage of a distillation unit is the ongoing cost of energy required for its operation.
Reverse Osmosis
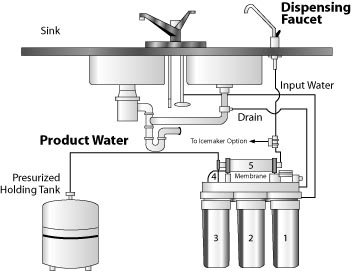 Figure 4:
Figure 4: Domestic reverse osmosis water treatment system.
(Recreated from: RAHA Water Treatment Corporation http://www.rahawater.com/rw400.htm).
Reverse osmosis (RO) is a membrane separation process that employs a very thin membrane with tiny pores to produce nearly pure water by separating out most of the dissolved minerals and suspended particles. Technically, this membrane is called a semipermeable membrane, because it allows pure water to pass through but retains any dissolved and suspended constituents. More formally, it is the process of forcing a solvent (pure water) from a region of high solute concentration through a membrane to a region of low solute concentration by applying pressure, a process that is opposite of osmosis and hence called reverse osmosis. Because only a portion of the water is forced through the membrane and the remaining water containing the unwanted materials is rejected, the system produces a substantial amount of waste water (Figure 4). Several kinds of reverse osmosis membranes are available. Each type has advantages and disadvantages. A membrane should be selected that will best remove the contaminants of interest. Sample and analyze the water before and after the treatment to determine the efficiency of the system.
Home RO units only treat about 5 to 10 percent of the flow through the unit and waste the remainder. Thus, if you are on a septic system, for every 1 gallon of treated water you may be discharging 9 to 19 gallons of water into your septic system. For this reason, RO systems are not feasible for treating all the water for a home. They are usually installed at the kitchen sink and connected to the household plumbing. A typical unit will treat between 5 and 20 gallons of water per day. Operation and maintenance costs of an RO unit are considerable - you have to replace the filtering membrane unit on a regular basis as recommended by the manufacturer.
Many RO units can provide up to 95 percent removal of a variety of inorganic and some organic chemicals. However, it is not effective against dissolved gases (e.g., hydrogen-sulfide, radon and trihalomethanes) or most volatile and semi-volatile organic contaminants, including some pesticides and solvents. A list of contaminants that could be treated effectively by RO membrane filters is presented in Table 5. This table is not an exhaustive list of contaminants that RO may remove, but rather lists those for which RO can be a practical method for treating household drinking water.
| Table 5. Contaminants removed by household reverse osmosis units. |
Inorganic Contaminants
(Ions and Metals) |
Aluminum, Barium, Bicarbonate, Cadmium, Calcium, Chloride, Chromium, Copper, Fluoride, Lead, Magnesium, Manganese, Mercury, Nitrate, Potassium, Radium, Selenium, Silica, Silver, Sodium, Sulfate, Uranium, Zinc |
| Microbials† |
Protozoan cysts, Cryptosporidium |
| Pesticides |
Endrin, Heptachlor, Lindane, Pentachlorophenol |
| Particles |
Asbestos |
|
* Many RO systems also include an activated carbon filter, which will allow for the reduction of additional contaminants that reverse osmosis units alone are not effective for (see section on Activated Carbon Filters).
† Although the RO membrane is capable of rejecting virtually all microorganisms, it can develop pinholes or tears that allow bacteria or other microorganisms to pass into the treated water. RO units alone are not recommended for treatment of bacteria and other microscopic organisms.
|
Activated Carbon Filters

 Figure 5.
Figure 5. Powdered and granular activated carbon.
(Sources: (top) Mrs. Pugliano, Flickr.com; (bottom) wikipedia.com)
Activated carbon (AC), also called activated charcoal, is usually derived from charcoal. It is a form of carbon that has been processed (activated) to make it extremely porous and thus have a very large surface area available for adsorption or chemical reactions. Most AC filters are made from raw materials such as nutshells, wood, coal, etc. Typically, 1 gram of AC may have a surface area of 1000 square-meter or more. Activated carbon is an adsorption medium, meaning that substances become bonded to the carbon and are tightly held there.
Activated carbon filtration is effective against unpleasant tastes, odors (hydrogen sulfide), residual chlorine, some metals like mercury, many organic compounds (like VOCs), some pesticides, gasoline, trihalomethanes, benzene and radon gas. A special type of AC filter called a Solid Block Activated Carbon filter can remove Cryptosporidium and Giardia cysts as well. However, AC filters cannot remove bacteria, nitrate, fluoride, chloride, hardness (calcium and magnesium) and most metal ions.
Filters are available in cartridge units for point-of-use treatment and in tank type units with granular activated carbon for point-of-entry treatment. When the absorption capacity of the activated carbon is used up, the media should be replaced for the filter to remain effective. A filter with a spent cartridge may be worse than no filter at all. Some recent studies show that if you don't replace your filter as recommended, bacteria can grow within the medium and become a source of drinking water contamination.
Solutions to Some Specific Water Quality Problems
Bacterial Contamination
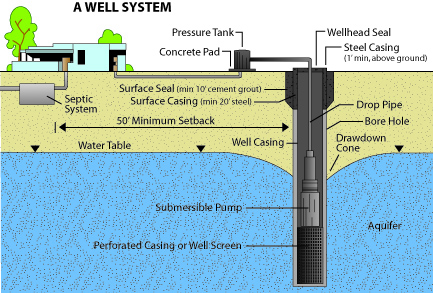 Figure 6:
Figure 6: Domestic well diagram with adequate well-head protection measures.
(Adapted from Arizona Department of Water Resources Well Owners Guide)
Water that is found to contain coliform bacteria is not safe for human consumption because the presence of coliform bacteria in well water indicates a possible source of entry for disease-causing organisms into the well.
If possible, locate the source of contamination and eliminate the problem at its source. Several origins of bacterial contamination are possible, the most common being surface water entering the well. This can usually be prevented by proper well construction and several wellhead protection measures (Figure 6) such as:
- Extending the well casing above ground level
- Sealing around the well casing with tight clay at least 10 feet deep from the surface (technically called grouting)
- Further sealing around the well casing with a concrete slab
- Sealing the top of the casing with a sanitary well cap
- Diverting all surface waters away from the well area
For further information about wellhead protection, refer to University of Georgia Cooperative Extension Circular 858-1, Protecting your Well and Well Head, available at your local Extension office and online at .
Other sources of contamination are septic tanks or sewage lines located too close to the well. Run-off or leaching from livestock operations can also contaminate wells. A recent study showed that the occurrence of contamination of well waters by coliform bacteria in Georgia is substantial, regardless of well types and age (Figure 7). That means much is left to be done to ascertain proper well construction, wellhead protection, and water testing and treatment to minimize the risk of water-borne diseases.
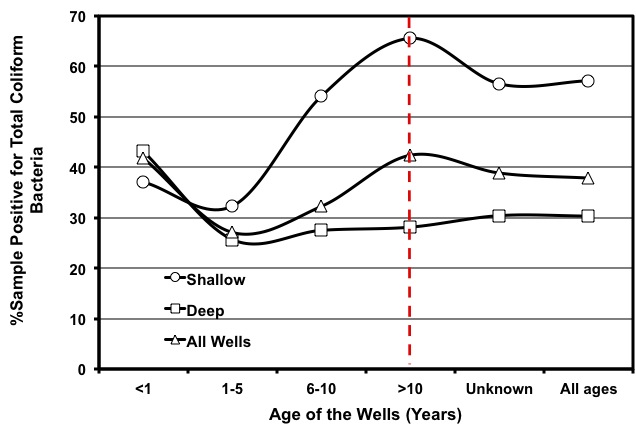 Figure 7:
Figure 7: Rate of coliform contamination in well waters of Georgia for various well ages and well types.
(Source: Saha et al., 2011)
If your water tests positive for coliform, you should take immediate steps to eliminate the source of contamination (if you can locate the source) and shock chlorinate the well and distribution system to get rid of the remaining bacteria. To shock chlorinate the well and distribution system, introduce a concentrated chlorine solution directly into the well and mix, then circulate the chlorinated water throughout the water system and allow it to stand overnight. Finally, flush the lines until the chlorine odor is no longer evident. For further details about shock chlorination procedures, refer to University of Georgia Cooperative Extension Circular 858-4, Disinfecting Your Well Water: Shock Chlorination, available at your local Extension office and online at: . Shock chlorination is required after construction, pump installation and after every major service operation. Anytime a pump is removed from a well and replaced, there is a chance of bacterial contamination.
If you cannot eliminate the source of contamination and/or experience recurrent contamination even after repeated shock chlorination, consider alternative sources of water. If this option is not feasible, you may have to resort to continuous disinfection of the water supply. Various water disinfection methods include chlorination, ultraviolet light and ozonation.
Chlorination
The most common, oldest and relatively inexpensive method of disinfecting water is chlorination, which is basically addition of bleach (chlorine solution) to the water. Chlorinators inject chlorine solutions into water systems at a controlled rate to ensure the desired chlorine concentration in the treated water. They are readily available and easily installed. Chlorine is a very effective disinfectant and oxidizing agent. With sufficient concentrations and adequate contact time, it kills pathogens, including bacteria and certain viruses, but does not kill Cryptosporidium, Giardia and some other microscopic organisms. It also readily combines with other components dissolved in water including iron, manganese, hydrogen sulfide, organic matter, ammonia, and organic color such as that from decaying peat moss. Thus, it also removes some bad odors, tastes and colors. However, chlorine can react with dissolved organic material and produce some toxic chemicals like trichloromethane (chloroform) that may be linked to human health hazards.
The part of the added chlorine used for initial disinfection and reaction with other dissolved components of water is called “chlorine demand of water.” The added chlorine that is left after being used up for satisfying the chlorine demand of water is called “free chlorine residual.” By maintaining a certain level of free chlorine residual you ensure a continued disinfection of the treated water until it is used. According to CDC (Centers for Disease Control and prevention), treated water should contain a free chlorine residual of at least 0.5 ppm to be safe (microbiologically) for drinking and no more than 2.0 mg/L to be free from any unpleasant taste or chlorine odor.
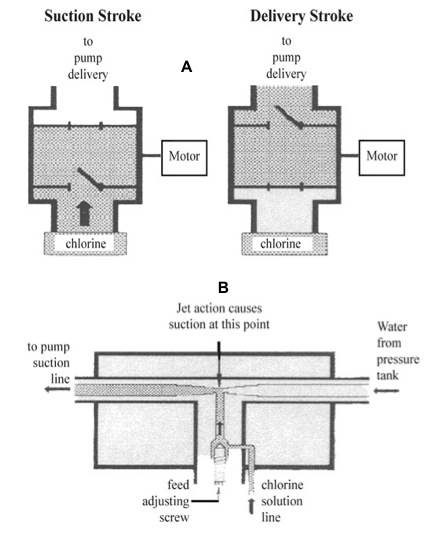 Figure 8:
Figure 8: (A) Chemical feed pump chlorinator. (B) Injection type chlorinator.
(Redrawn from Chlorination of Drinking Water, Wagenet, L, and Lemley, A., Cornell Cooperative Extension, New York State College of Human Ecology.)
The time required for chlorine to effectively destroy bacteria is known as the contact time. A contact time of at least five minutes is recommended, provided a free chlorine residual of 0.5 to 1.0 ppm is maintained. In order to provide adequate contact time, install an intermediate storage tank with a theoretical water detention time of 10 to 15 minutes. For instance, if the pump capacity is 10 gallons per minute then the storage tank should hold at least 100 gallons to ensure 10 minutes of desired contact time. The pressure tank is usually not adequate for providing extra contact time because when water is being pumped and drawn at the same time, the fresh water may by-pass the water already in the pressure tank.
Household laundry bleach (sodium hypochlorite, NaOCl) containing 5.25 percent available chlorine can be used as a source of chlorine. Calcium hypochlorite Ca(OCl2), in powder or tablet form, may also be used as a concentrated source of chlorine to prepare a stock solution. Mix according to label directions to obtain the proper concentration of chlorine. After mixing, inject only the clear solution. Any sedimentation in the bottom of the container should be discarded. A fresh solution should be prepared at least once per week. Follow the instructions provided by the manufacturer functioning and maintenance of the chlorination unit.
Use a free chlorine test kit to periodically check the free chlorine residual of the treated water. These test kits are usually available from water treatment system suppliers. Information about borrowing and testing chlorine in your water using a test kit are available at your local Extension office and online at .
Ultraviolet Light
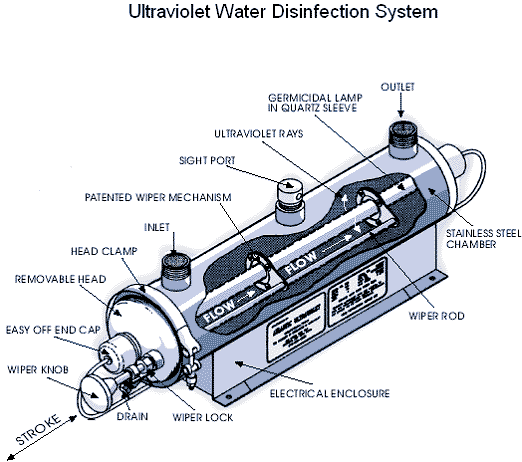
Figure 9: Ultraviolet water treatment system (Source: thewatertreatmentplant.com).
Ultraviolet (UV) light has been used to disinfect public water supplies for more than 75 years, but home UV systems have become available only recently. This type of water treatment uses a low pressure mercury arc lamp that emits UV light to kill pathogens in the water. The equipment consists of ultraviolet lights surrounded by tubes through which the water must pass. Bacteria exposed to the UV light are destroyed or inactivated. The principal advantage to UV treatment over chlorination is that it disinfects water without using any chemicals, and thus does not impart any taste or odor to the water. Furthermore, some water-borne microorganisms are chlorine-resistant, so chlorination may not be adequate to rid your tap water of dangerous levels of some pathogens.
Although UV treatment is effective, disinfection only occurs within the unit. No disinfection occurs beyond the treatment unit to kill microorganisms that survived or were introduced in the system after UV treatment. Unlike chlorination, where easily measured “free chlorine residual” can be used as an indicator of continued bacteria-free water, a UV unit requires expensive microbiological tests from time-to-time to verify adequate performance.
UV light kills bacteria, viruses and some cysts. It does not kill either Giardia lamblia cysts or Cryptosporidium parvum oocysts, both of which must be removed by microfiltration or distillation. UV is not recommended if the untreated water has a coliform content exceeding 1,000 total coliforms or 100 fecal coliforms per 100 milliliters.
The bacteria must be exposed to the UV light for a certain length of time to ensure disinfection. Color, turbidity, scale build-up due to hard water, and organic impurities in water interfere with the transmission of the ultraviolet energy and may reduce the disinfection efficiency to unsafe levels. Ongoing costs of energy and UV-lamp replacement should be considered before installing a UV disinfection system.
Emergency Measures
If you suspect bacterial contamination of a water supply, take emergency measures to make sure the water is safe for consumption until permanent solutions can be employed. If alternate water sources are not available, boil or shock chlorinate the water before drinking.
Boiling the water vigorously for at least two minutes will destroy harmful organisms. Once the water is cooled, it must be protected from recontamination. The boiled water will have a “flat” taste that you can eliminate by aeration.
In order to shock chlorinate drinking water, add 1/8 teaspoon (or eight drops) of regular, unscented, liquid household bleach to 1 gallon of water, stir it well and let it stand for 30 minutes. Although the water may have an objectionable chlorine taste, it will be microbiologically safe to drink. You can reduce the chlorine taste by heating the water or allowing it to stand for a longer period of time.
Hard Water
Water hardness is due to the presence of certain dissolved minerals, primarily calcium and magnesium. Hard water can cause scale build-up in hot water pipes, water heaters and plumbing fixtures, thereby increasing the costs of heating water and reducing the life of the appliance and plumbing system. Hard water minerals also interfere with the cleaning action of soaps and detergents, forming film on skin, clothing and fixtures. However, there is no known health hazard due to drinking hard water.
Water hardness is reported in one of two ways, either as milligrams per liter (parts per million) as calcium carbonate or as grains per gallon. The most common method used on water test reports is grains per gallon. The degree of water hardness is classified as follows (Table 6):
| Table 6. Classes of water hardness. |
| Water Hardness |
Grains per Gallon |
Parts per Million (ppm) |
Soft
Slightly Hard
Moderately Hard
Hard
Very Hard |
0 to 0.99
0.99 to 3.5
3.5 to 7
7 to 10.5
more than 10.5 |
0 to 17
17 to 60
60 to 120
120 to 180
more than 180 |
Water with hardness exceeding about 7 grains per gallon or approximately 120 mg/L (parts per million) may interfere with the cleaning capacity of soaps and detergents, thereby affecting laundering, washing dishes, bathing and personal grooming.
The most common method of removing hardness for an individual water system is ion-exchange, also called water softening. A household water softener contains a “mineral tank” containing a column of material called “cation exchange resin beads.” As the hard water passes through the mineral tank, the column of resin beads releases sodium into the water and adsorbs or removes calcium and magnesium from the water. As the exchange continues, the column becomes saturated with calcium and magnesium. The column is then regenerated by back-washing the resin with a concentrated solution of rock salt (sodium chloride) prepared in another tank called the “brine tank.” The excess salt, calcium and magnesium solution is washed (rinsed) out of the resin, and the resin is again ready to exchange sodium for calcium and magnesium.
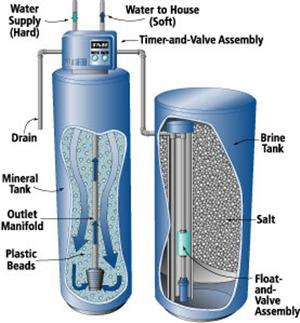 Figure 10:
Figure 10: Automatic water softener.
(Source: www.mechanicalwritings.com)
Water softeners are available with automatic, semiautomatic or manual regeneration. Fully automatic units regenerate on a predetermined schedule (usually controlled by a clock) and return to service automatically. Semiautomatic units are started manually, but otherwise operate automatically. With manual units, all steps (backwashing, brining and rinsing) are performed manually.
Turbid water, or water with iron or bacterial slimes, can clog a water softener. If these impurities pose a problem, filter them out before the water enters the softener or clean the softener manually periodically.
Water softeners are sized according to the water hardness and the daily water requirements of the household. Usually, softened water is supplied only to the bathtub, lavatories, kitchen sink and laundry. Water for the toilet and for the lawn, garden and other nonhousehold uses is usually not softened. This reduces the load on the unit and thus the frequency of recharging. As a rule the softener should be large enough to last at least three days between regenerations.
In softening water with an ion-exchange softener, sodium is added to the water. For this reason, people on a restricted sodium diet (for example, individuals with high blood pressure) should consult with their health professionals before drinking softened water long-term.
Nitrate
Nitrate (NO3) is a primary form of nitrogen (N) for plant growth. Nitrate fertilizers are used extensively in agriculture. If properly managed, the use of nitrogen for agriculture does not pose a particular health problem by contaminating drinking water supplies. However, when more nitrogen is added to the soil than the plants can use, excess nitrate can leach into groundwater supplies.
Human infants, infant monogastrics (such as baby pigs and chickens) and ruminant animals (such as cows and sheep) have bacteria in their digestive systems that convert nitrate to nitrite, a very toxic substance. When nitrites are absorbed into the blood, they make the hemoglobin (red blood pigment carrying oxygen) incapable of releasing the oxygen and mild symptoms of asphyxiation (a condition of deficient oxygen supply to the body) appear. Infants under six months of age are most affected by excess nitrates in the water. They may eventually develop a condition called methemoglobinemia (blue baby syndrome), which causes a bluish color around the lips, spreads to the fingers, toes and face, and eventually covers the entire body.
Nitrate test results are usually expressed as nitrate-nitrogen (NO3-N); however, some laboratories may report the amount as nitrate (NO3). Nitrate-nitrogen is just the nitrogen portion of the nitrate ion: Nitrate (mg/l) = Nitrate-Nitrogen (mg/l) x 4.427. Because of the difference between the number expressed as nitrate-nitrogen or as nitrate, it is essential to use the correct scale to interpret your water test report. If your test report is unclear whether the number reported is nitrate or nitrate-nitrogen, check with the laboratory.
A water quality standard of 10 milligrams per liter nitrate-nitrogen (mg/l NO3-N) has been set for human consumption and 100 mg/l NO3-N for livestock.
If you find your water supply is contaminated with nitrate, take steps to locate the source of contamination. Nitrates can enter the ground water through fertilizer application, feedlot runoff or septic systems. Make sure the well is properly cased, the wellhead is adequately protected and that surface water is diverted away from the well (see the section on coliform bacteria in this publication). Also, the well should be at least 50 feet away from a septic tank and at least 100 feet away from a septic tank absorption field because both are potential sources of contamination. A test for bacteria is suggested when well water shows high levels of nitrate, and vice versa. Septic system leakage is the most likely source of nitrate contamination in your well.
Once a water supply becomes contaminated with nitrate, it is very difficult and costly to treat. It may be practical to treat only household drinking water, but it would be very costly to treat the large volumes of water consumed by livestock or used for other household uses. The possible methods of reducing or removing nitrates from water are: demineralization and anion exchange. Demineralization removes nitrates and all other minerals from the water and can be accomplished in two ways: distillation and reverse osmosis (described earlier in this publication).
The anion exchange system operates on the same principle as a water softener. The water passes through a column of resin beads in which nitrate and sulfate ions are exchanged for chloride ions. The resin is recharged by backwashing with a brine solution (sodium chloride) just as with a water softener. Since this process also removes sulfates, any sulfate in the water supply may interfere with nitrate removal. The resin may also make the water corrosive, requiring the water to go through a neutralizing system after going through the anion-exchange unit.
Acidity
In ground water, the cause of acidity is usually free carbon dioxide in the water. The gas may come from decaying organic matter or it may be carried down from the air by rainwater. Some sand aquifers have naturally occurring acidic water because they do not contain minerals that buffer the pH. In some cases, especially in mining areas, water may contain free mineral acid—hydrochloric, sulfuric or nitric.
Acid water causes problems by corroding the metal parts of water systems. The first symptoms of acid water usually appear in the form of stains in toilets, sinks and other fixtures. The color of the stains will depend on the kind of metal being attacked—green or blue stains from copper and red or brown stains from iron. If the corrosion is allowed to continue, the metal will slowly be eaten away, and the system will ultimately fail. Lead in water is also usually the result of water acidity, which dissolves lead from soil, rock or from the plumbing system. Lead piping and lead solders in copper pipes are found in many houses built prior to 1986. Lead is a primary contaminant in drinking water that causes some serious health consequences if water containing lead above the MCL is consumed for a considerable period of time. Concentration of lead in water exceeding the MCL, however, does not cause any abnormal color, odor or taste.
The acidity or alkalinity of water is measured on a scale known as pH. The pH can vary from 0 to 14, with a pH of 7 being neutral. If the pH is below 7, the water is acidic; above 7, it is alkaline. Ideally, the pH of domestic water should be between 6.5 and 8.5 pH. Values below 6.5 usually indicate corrosive water; if the pH is above 8.5, the water is usually hard.
The most obvious solution for treatment of acid water is to neutralize the acidity. One of the simplest ways to do this is to install a neutralizing filter, which contains a bed of material such as calcium carbonate or magnesium oxide. As the water passes through the filter, the acid is neutralized and a small amount of the bed is dissolved. Neutralizing filters must be backwashed periodically because they also serve as mechanical filters to remove solid particles from the water. Also, from time to time the bed needs to be replenished to replace material that was dissolved. Acidic water can also be neutralized by injecting a solution of soda ash (sodium carbonate) in the water supply with a chemical feed pump.
Where acidity is the only problem, the neutralizing filters are usually the best approach. However, if the water contains high levels of iron or manganese or if the water requires disinfection, the chemical feed pump is often used, since chlorine (for disinfecting) and soda ash may be mixed in a single solution and fed into the water system with the same pump unit.
Turbidity
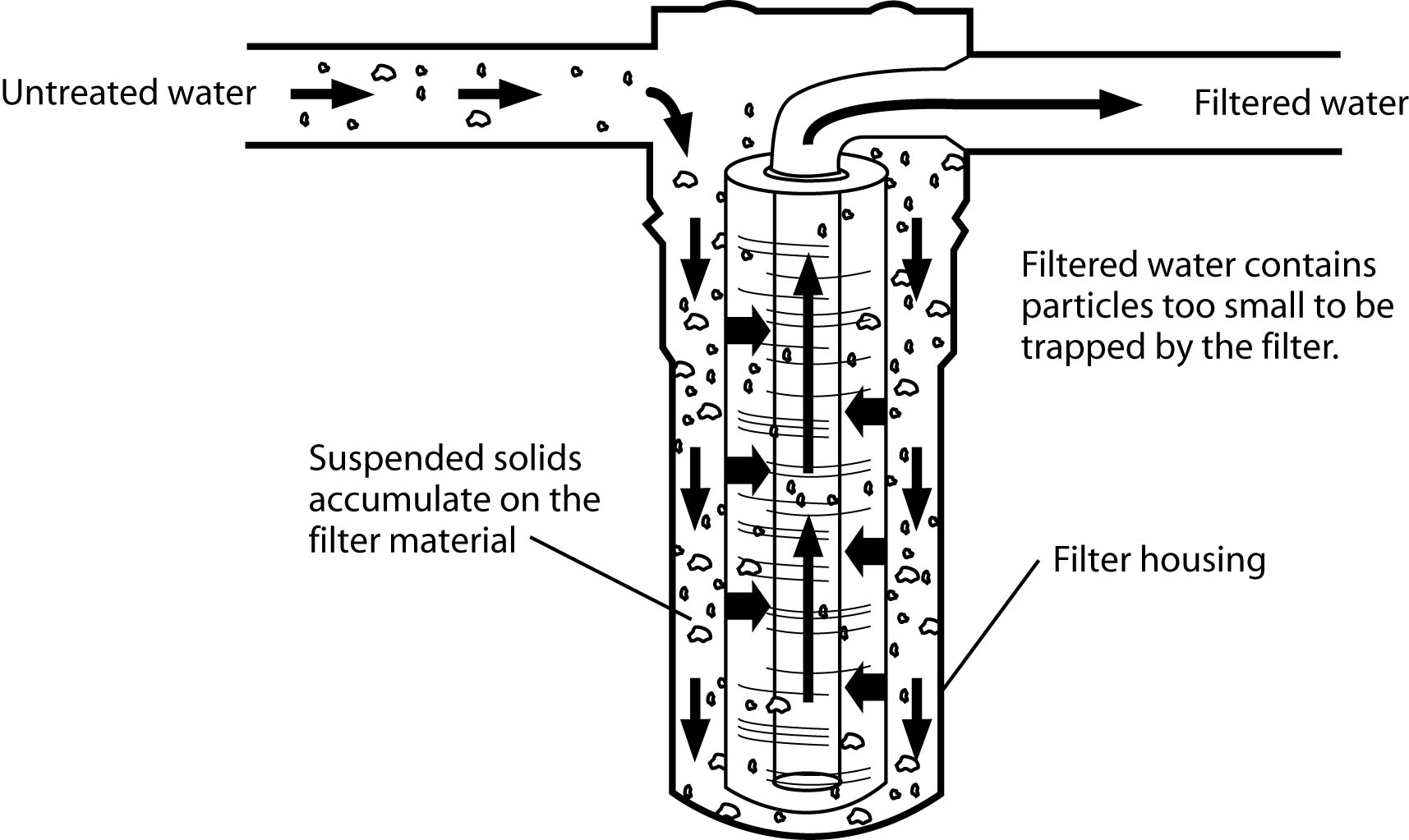 Figure 11:
Figure 11: The sediment filtration process with cartridge filter.
(Redrawn from Wagenet, L, K. Mancl, and M. Sailus, 1995. “Home Water Treatment,” Northeast Regional Agricultural Engineering Service, Cooperative Extension, Ithaca, NY)
The presence of suspended materials such as clay, silt, finely divided organic material, plankton, etc. absorb or reflect light and result in cloudy or muddy water. Turbidity is measured based on light transmission through water; light transmission is lower through turbid waters than clean waters. Even though surface waters are frequently turbid, turbidity problems with well waters is also found, especially when the well screen is not sized correctly or the water table drops and the water cascades into the well. If the well is drilled through fractured rock you can have turbidity problems - and the grit can damage your pump.
The major problem with turbidity is aesthetics, but in some cases suspended matter can carry pathogens with it. Large amounts of organic matter can also produce stains on sinks, fixtures and laundry. Organic matter in water may also produce colors, unpleasant tastes and odors. These tastes and odors will affect not only drinking water, but also the foods and beverages prepared with the water. If turbidity is greater than 5 turbidity units (NTU - Nephlometric Turbidity Units), be aware of possible bacterial contamination.
Mechanical filtration is required for the removal of turbidity. Two different types of filters may be used, either individually or in combination. One is the sand filter and the other is the cartridge filter.
The sand filter consists of a tank containing an 18- to 24-inch-deep bed of fine sand on top of a layer of fine gravel. The water passes through the sand bed to filter out suspended particles. The filter must be backwashed periodically to clean the beds and to flush away accumulated sediment. The sand filter is effective in removing all but extremely fine particles.
The cartridge filter is usually much smaller than the sand filter and is often used in the water line to a specific tap. These filters use media that is formed or molded into more or less rigid cartridges. Most cartridges are designed to be replaced when they become clogged with accumulated solids. The cartridge filter is very effective in removing extremely fine particles. It is always better to remove the exhausted filter than leave it in and not replace it when needed because bacteria can grow in the filter material.
A sand filter is best for removing heavy loads of suspended particles, while the cartridge filter may be used as secondary filtration at the point of use to remove very fine particles not removed by the sand filter. If the turbidity is relatively low, the cartridge filter may be all that is needed.
Often, some new wells may initially yield turbid water due to high levels of suspended solids added during the drilling process. These solids can usually be removed by an initial period of continuous pumping, called well “development.” Cartridge filters can be used initially and after the well clears up, although the cartridge should be removed to bypass the system.
Iron and Manganese
 Figure 12:
Figure 12: Typical stains caused by iron in water.
(Source: smoMashup, Flickr.com)
Iron and manganese are common problems encountered with private water systems in Georgia. Iron gives water a bitter, metallic taste, stains clothes and fixtures and promotes the growth of iron bacteria in plumbing. Manganese causes similar problems and can be removed by the same procedures used for iron. The secondary MCLs are 0.3 ppm for iron and 0.05 ppm for manganese. For further information, refer to University of Georgia Cooperative Extension Circular 858-11, Your Household Water Quality: Iron and Manganese, available at your local Extension office and online at: .
Iron can be present in two forms: oxidized or reduced. The reduced form of iron is present in an oxygen-free environment, such as ground water. This form of iron is water soluble and colorless. When reduced iron is oxidized, it forms a red or yellow insoluble precipitate. It is the oxidized form of iron that stains plumbing fixtures and clothing. Iron can be oxidized by exposure to air or oxidizing agents such as chlorine, permanganate and manganese green sand used as filter media in the water treatment industry.
Iron bacteria can be a problem with iron concentrations as low as 0.1 ppm. Iron bacteria form slime growths or gelatinous masses in plumbing and use the iron in the water as an energy source. They do not cause disease but can be a real nuisance. Heavy growths can completely plug pipes, but usually break loose during periods of high water flow to produce slugs of dirty iron- or manganese-laden water with obnoxious tastes and odors. A reddish-brown slimy growth in a toilet flush tank is a good indication of the presence of iron bacteria in a water system. Iron bacteria can be removed by shock chlorinating the well and whole plumbing system as described earlier. If the problem persists, continuous chlorination may be necessary.
Several methods are available for removing iron and manganese from water. The best method for you will depend on the concentration and whether or not the iron or manganese is in the oxidized or reduced form.
High-capacity Water Softeners for Iron and Manganese Removal
In addition to removing calcium and magnesium in exchange for sodium, water softeners will remove the reduced forms of iron and manganese. Water softeners are effective up to 5.0 ppm of combined iron and manganese. It is important to note that water softeners are only effective on reduced iron and manganese (which are soluble forms); oxidized forms (solid particles or precipitate) will foul the unit. If oxidized forms are present, they should be removed by a sand filter before the water reaches the softener.
Oxidizing Filter
Oxidizing filters are recommended when:
- Iron or manganese concentration is too high for a water softener
- Much of the iron is oxidized
- Iron removal without softening is desired
The media in these filters is capable of oxidizing iron or manganese to the insoluble state and removing the precipitated matter all in the same tank. This is a point-of-entry treatment method. The two major types of filter media commonly used are manganese greensand and manufactured zeolite coated with manganese oxide.
These filters must be backwashed periodically to flush the accumulated deposits. When the oxidizing capacity of the filter medium declines substantially, the medium has to be regenerated with a weak potassium permanganate solution to restore the oxidizing capacity. These filters are effective with combined iron and manganese concentrations up to 15 ppm. The pH of the water should be above 7.0 for the filter to be most effective.
Chemical Oxidation Followed by Mechanical Filtration
Chemical oxidation followed by mechanical filtration is also a point-of-entry treatment method that oxidizes dissolved iron and manganese into solid particles that are subsequently filtered out of the water. This is the accepted method of iron and manganese removal when their combined concentrations are greater than 10 ppm. This treatment is particularly valuable when iron is combined with organic matter or when iron bacteria are present. This method destroys iron bacteria, which would foul a water softener or oxidizing filter.
With this type of treatment system, an oxidizing chemical is added into the water by a small feed pump that operates when the well pump operates. Addition of chemical is typically done just before the water enters a storage tank. Generally, the oxidation process requires at least 20 minutes of retention time in the storage tank. Sometimes the chemical is added to the well to control the iron bacteria. Dissolved iron, manganese and hydrogen sulfide are oxidized, forming solid particles. Oxidizing chemicals that can be used are:
- Chlorine
- Potassium permanganate
- Hydrogen peroxide
In addition to its oxidizing effect, a special advantage of using chlorine is its bactericidal effect. Iron and manganese bacteria, along with other bacteria, are destroyed. However, iron oxidation by chlorine is most effective if water pH is 6.5 to 7.5. Consequently, chlorination is not recommended for treatment of high levels of manganese because a pH level of 9.5 or greater is required for complete manganese oxidation. In contrast, potassium permanganate can oxidize manganese at pH levels of 7.5 or higher and is also an effective method of oxidizing organic iron. The solid particles formed in the oxidation step can be subsequently removed through a mechanical filter (a sand filter, for example).
Sulfur Water
A strong rotten egg taste or odor usually indicates the presence of hydrogen sulfide in the water. Water containing this gas is commonly known as “sulfur” water. Not only does it have a bad taste and odor, it can also corrode iron and other metals and cause stains in plumbing fixtures. Treatment is usually needed if there is more than 1.0 ppm of sulfur in the water.
When only small amounts of hydrogen sulfide are involved (6 ppm or less), an iron-removal filter will remove the sulfur satisfactorily. If concentrations are higher (greater than 6 ppm), the hydrogen sulfide can be removed by chlorination followed by filtration through a sand filter. The chlorine will oxidize the sulfur, changing it to an insoluble form, and the filter will remove the suspended particles. The chlorine will also kill sulfur bacteria if they are present.
If your water system has not been used for a while, if the well has not been pumped or your storage tank has not been flushed, stagnant water can generate a rotten egg odor. Flushing your system can reduce the odor.
Arsenic and Uranium
Arsenic (As) is a trace element of concern in some well waters in Georgia. It naturally occurs in some aquifer sediments. Test results from ½ûÂþÌìÌÃ's water laboratory and Georgia's Environmental Protection Division revealed that some well waters had arsenic concentrations above the MCL of 10 ppb, which the EPA considers unsafe for drinking. These samples were widely distributed geographically in Camden, Irwin, Tift, Bibb and Lowndes counties. For further information about arsenic occurrence and health concerns of arsenic exposure, refer to University of Georgia Cooperative Extension Circular 858-12, Your Household Water Quality: Arsenic in Your Water, available at your local Extension office and online at .
There are typically two forms of arsenic in water: “arsenic-III” and “arsenic-V,” but arsenic-III often predominates in the groundwater. Arsenic-III is much more toxic to humans and is relatively difficult to remove compared to arsenic-V. Therefore, any water filtration should remove both forms of arsenic. The preferred water treatment is to remove arsenic from all water entering the house using granular ferric oxide, titanium or hybrid adsorption media that contains iron-impregnated resin. These systems effectively remove both “arsenic-III” and “arsenic-V.” A smaller and lower-cost filtering system at the point-of-use can be an alternate choice, which would provide 2 quarts of treated water per minute, enough for drinking and cooking in an average household. For further information about arsenic removal, refer to the University of Georgia publication Removal of Arsenic from Household Water, available at your local Extension office and online at: .
Uranium (U) is another trace element that has been found in some well waters exceeding the EPA MCL of 30 ppb. Uranium is naturally occurring in certain kinds of aquifer bedrock. In Georgia, waters that could be high in uranium are located primarily in the Piedmont and Blue-Ridge regions located in the northern part of the state (above the “Fall Line”) and supplied by wells deeper than 100 feet in granitic bedrock. Levels of uranium above 30 ppb have not been found in shallow wells or surface water. An affordable home water-treatment option is a “point-of-use” reverse osmosis (RO) system that produces 5 to 20 gallons of drinkable water per day and removes 90 to 99 percent of uranium.
Independent Validation and Certification of Water Treatment Equipment
There are numerous water treatment systems on the market that claim to effectively handle various practical water quality issues. Well owners may get confused by competing, often contradictory, statements and have difficulty determining which claims are accurate. The importance of validating the accuracy of advertising literature of the product, followed by certification by an independent third party, is paramount. If a treatment system is certified by a reputable third party, well owners can be assured that the product will perform as specified. In the water treatment industry, NSF International () and the Water Quality Association (WQA, http://www.wqa.org) are two recognized organizations involved in independent validation and certification of various products.
- National Sanitation Foundation (NSF) International is a nonprofit organization dedicated to solving health and environmental problems. They provide product certification and listing services that show the results of third-party evaluations, testing and inspection programs performed on water treatment units. Their certification of a treatment system means that it not only performs as claimed, but also that the advertisements associated with the product in the market are accurate and true. Products are tested on an ongoing basis to make certain that companies continue to produce products that perform as advertised. Thus, the NSF is an unbiased source of consumer protection against fraudulent dealers. However, an NSF seal only means that the device has been tested according to NSF protocols and passed. This does not always mean that the product will continue to perform for extended periods of time.
- WQA is a self-governing body of manufacturers and distributors of water treatment products. The association provides educational materials to the consumer, a product testing service for the industry and promotes the use of treatment equipment. The WQA has developed a “Gold Seal” program for member products that have been tested and approved by the association. The WQA tests prototype water treatment equipment and awards the “Gold Seal” only to those systems that have met or exceeded industry standards for contaminant reduction performance, structural integrity and materials safety. WQA attempts to police the industry to eliminate firms that sell products that do not perform as per advertising claims.
The NSF program established performance standards that must be met for endorsement and certification. The WQA program uses the same NSF standards and provides equivalent American National Standards Institute (ANSI) accredited product certifications. Though these certifications and validations should not be the only criteria for choosing a water treatment system, they are helpful to ensure effectiveness of the system. There are currently seven ANSI/NSF standards relating to water filtration and treatment devices, each one designed for a specific type of product.
- STANDARD 42: Drinking Water Treatment Devices - Aesthetic Effects
- STANDARD 44: Cation Exchange Water Softeners
- STANDARD 53: Drinking Water Treatment Devices - Health Effects
- STANDARD 55: Ultraviolet Microbiological Water Treatment Systems
- STANDARD 58: Reverse Osmosis Drinking Water Treatment Systems
- STANDARD 62: Drinking Water Distillation Systems
- NSF/ANSI 177: Shower Filtration Systems - Aesthetic Effects
You should know exactly what a specific certification standard stands for if the certification is to be useful. The NSF site has online comparisons of many of the products they certify. Check out the NSF site, and use it as one of your guides in choosing a reliable product by selecting “Drinking Water Treatment Units” and then entering one or more of the following pieces of information:
- A company name you are interested in investigating (if any)
- Brand name/Trade name/Model you are interested in investigating (if any)
- The product standard (42, 44, 53, etc.)
- Product type (countertop filter, under counter filter, etc.)
- Facility location (e.g., Georgia)
Other organizations like the Better Business Bureau, County Extension Offices, and Consumer Reports can provide additional unbiased information to prospective treatment equipment buyers. Because these organizations often deal directly with consumers, they know the success and failure rates of various devices and may provide buyers with information like company reputation, warranties, and facts on water treatment. A wise consumer should seek out one or more third party opinions before selecting a company or device (Table 7).
| Table 7. Sources of information and product evaluators for drinking water treatment equipment. |
|
Non-profit and Independent
National Sanitation Foundation, 3475 Plymouth Road, Ann Arbor, MI 48106
(), 800-NSF-MARK (800-673-6275).
American Water Works Association, 6666 W. Quincy Ave., Denver, CO 80235
(), 800-926-7337.
American Water Resources Association, P.O. Box 1626, Middleburg, VA 20118 ), 540-687-8390.
Consumer Reports
().
Better Business Bureau, 4200 Wilson Blvd, Suite 800, Arlington, VA 22203
(), 703-276-0100.
|
|
Government and Educational Institutions
½ûÂþÌìÌà County Extension, Local Offices
(), 1-800-ASK-½ûÂþÌìÌÃ1.
USDA, Cooperative Extension System,
14th & Independence Ave., Room 3346 South, Washington, DC 20250-0900
().
U.S. Environmental Protection Agency Office of Water,
1200 Pennsylvania Avenue, N.W. Washington, D.C. 20460
().
National Technical Information Service,
U.S. Dept. of Commerce, 5301 Shawnee Rd, Alexandria, VA 2231
(), 800-553-6847.
Georgia Department of Health, Local Offices
()
|
|
Industry or Manufacturer Organizations
Water Quality Association, 4151 Naperville Road, Lisle, Illinois 60532-3696
(), 630-505-0160
|
Cautionary Note: In researching water purification devices, it is often found that many companies state on their literature some vague claims like, “Tested to NSF standards” or “Tested and Certified in Accordance with NSF/ANSI Standards 42 and 53” (with the NSF logo displayed). Such statements do not ascertain that the product is certified by NSF because the answers to the following pertinent questions are unknown:
- Tested by which organization?
- Tested how often?
- Who backs up that claim and provides certification?
Water treatment systems that carry such vague statements in their advertisement literature are often not found in the list of certified products available online at the NSF website.
Questions to Ask When Purchasing Water Treatment Equipment
With the recent expansion of the home water treatment industry, new products are constantly being introduced with claims of solving a variety of water quality problems. Consumers often make costly decisions about water treatment equipment without being well informed. In many cases, people simply do not know what questions to ask to ensure a worthy investment. The following are some important questions that you should ask water treatment professionals to determine the treatment system that you need. The extent to which the manufacturer or distributor is willing to provide answers can assist the consumer in making an informed choice.
1. What exactly does the analysis of the water by the treatment professional show? Are health hazards indicated? Should more testing be done?
Many water treatment companies include in their services free in-home testing of the water. Not all contaminants can be evaluated this way; for example, arsenic, uranium or organics, which have been associated with serious health problems, must be analyzed in a laboratory with sophisticated equipment. The consumer must question the validity of analyses claiming to determine more than some basic water quality constituents such as hardness, pH, iron and sulfur. You should have a qualified individual examine your test results before making any purchasing decisions based on free water tests. The county health department, county Extension office, or water testing laboratory manager can help you evaluate water test results.
2. How long has the company been in business, and is there a list of referrals the consumer can contact?
Make sure the company is reputable and established. Ask the company for referrals and contact the referrals to find out customer satisfaction. Talk to your local health department to see if they have had any experience, good or bad, with the company.
3. Is the product tested and certified by the National Sanitation Foundation (NSF) or the Water Quality Association (WQA) for performance?
A product tested by an independent testing agency such as NSF or WQA will have a seal as shown below, indicating that it meets industry standards for water treatment performance.
Do not buy a device that does not have a seal indicating it meets industry standards.
4. Was the product tested for the specific contaminant in question and over the advertised life of the treatment device under household conditions (tap water, actual flow rates and pressures)?
If no test results are available, consider purchasing a different brand. You should examine test results of the device carefully to determine if the manufacturer's claims are realistic. For example, some reverse osmosis units are often claimed to be effective in removing arsenic and carry an NSF certified seal. That certification is only for Arsenic-V, but not for Arsenic-III.
When both Arsenic-V and Arsenic-III are found in the well waters in Georgia, the reverse osmosis unit will not be a practical solution to the arsenic contamination.
5. Is a second opinion on treatment procedures and equipment necessary?
Consider getting a second opinion on recommended water treatment equipment. Check with at least one additional dealer to see what treatment procedure and equipment is recommended and ask questions. Compare at least two brands and consult other references.
6. Does the specific water problem require wholehouse treatment (point of entry) or a single-tap device (point of use)?
The water treatment device selected depends on the contamination problem. For example, disinfection by chlorination or an ultraviolet system is generally done by a POE system, where all water used in the house should be treated. For most contaminants, treatment of only drinking and cooking water will provide safety at a reduced cost.
7. Will the manufacturer include follow-up water testing in the purchase price to ensure the equipment is working properly after a month or two?
Testing the water a month or two after the equipment is installed will assure the homeowner that the unit is accomplishing the intended treatment. You should ask for a written guarantee that the device will correct the specific problem, as documented by water testing a month or two after installation.
8. Will the device produce enough treated water to meet daily household requirements?
The maximum flow rate should be adequate for peak home use. You may also need to check whether your water system has the capacity for the treatment unit's requirement to perform adequately. For example, be sure you have adequate pressure in your system for a reverse osmosis unit.
9. Is there a shutoff system in case of malfunction? Is there an indicator light or alarm to indicate a problem?
Some units have shutoff systems and indicators to prevent you from consuming untreated water.
10. Does the device require maintenance? How do I know when maintenance is necessary?
Devices such as activated carbon units, reverse osmosis (RO) units, iron filters, etc. require regular maintenance. Make sure you understand the cost and effort necessary to properly maintain the equipment. Know how to contact company representatives if you have any questions after the device is installed.
11. Can I install the device and perform required maintenance myself or do I have to rely on company service?
You may be able to save a great deal of money with do-it-yourself equipment, but make sure the job is done right. Your money is wasted if equipment is not working properly. Find out if the warranty is voided if you perform maintenance on the device.
12. What are the total costs for purchase and maintenance, including labor for installation and service?
Watch for hidden costs such as installation fees, regular maintenance fees, equipment rental fees or costs associated with disposal of reject water or spent cartridges. Also ask about the electrical usage of the device.
13. What is the expected lifetime of the device? How long does the warranty last and what does it cover?
Consider the long-term cost of replacement or repair when making your purchase decision. Know all the requirements to keep the warranty in effect.
These questions serve as guidelines for consulting with water treatment equipment representatives. It is wise to shop around and get the best deal possible on the water treatment equipment you really need. Always get all guarantees and promises in writing and know how to contact the company selling you the equipment. It is always beneficial to work with a water professional while considering any water treatment system.
Summary
In the past, if you lived in a rural area, you were forced to live with whatever water supply was available, no matter how poor the quality of water was.
Often, the only alternative was to move. We now have methods available to treat almost any water quality problem. We also understand more about and are more aware of toxic effects associated with impurities in water. As a result, health problems associated with poor water quality can be minimized or prevented if water testing is done on a regular basis and appropriate measures for well maintenance and water treatment are taken in consultation with laboratory and water treatment professionals.
Sources
A Guide to Home Water Treatment, MSU Extension Water Quality Bulletins-WQ219201, Michigan State University Extension, Lansing, Michigan, 1997.
Agri-Facts, Reverse Osmosis Water Treatment. Agdex 716 (D36), Alberta Agriculture and Rural Development, AB, Canada.
Alex, C and R. L. Mahler. 2006. Iron in Drinking Water. PNW 589, University of Idaho Extension, the Oregon State University Extension Service, and Washington State University Extension. Available online at:
Bach, A. and Lundstrom, D. 1988. Household Water Treatment, 25 HF & E-2. NDSU Extension Service, North Dakota State University, Fargo, ND.
Bergsrud, F., B. Seelig, and R. Derickson. 1992. Treatment Systems for Household Water Supplies: Chlorination, AE-1046. North Dakota State University. Available online at: .
Daniels, B. and N. Mesner. 2005. Drinking Water Treatment Systems. Utah State University Cooperative Extension. Available online at:
David L. Varner, D.L., S. Skiptony, D. H. Paul and J. Jasa. 1996. Drinking Water: Iron and Manganese, G96-1280. Institute of Agriculture and Natural Resources at the University of Nebraska-Lincoln.
DeSilva, F. 2000. Exploring Multifunctional Nature of Activated Carbon Filtration. Water Quality Product Magazine, 17, January 2000. Available online at:
Dvorak, B.I. and S.O. 2008. Drinking Water Treatment: Distillation, G1493. Institute of Agriculture and Natural Resources at the University of Nebraska-Lincoln.
Dvorak, B.I. and S.O. 2008. Drinking Water Treatment: Sediment Filtration, G1492. Institute of Agriculture and Natural Resources at the University of Nebraska-Lincoln.
Dvorak, B.I. and S.O. Skipton. 2008. Drinking Water Treatment: Reverse Osmosis, G1490. Institute of Agriculture and Natural Resources at the University of Nebraska-Lincoln. Available online at: .
Dvorak, B.I. and S.O. Skipton. 2008. Drinking Water Treatment: An Overview. EC703. Institute of Agriculture and Natural Resources at the University of Nebraska-Lincoln. Available online at: .
Hassinger, E., T. Doerge, and P.B. Baker. 1994. Water Facts: Number 6 Reverse osmosis units. Arizona Cooperative Extension, The University of Arizona, College of Agriculture, Tucson, Arizona.
Healthy Drinking Waters for Massachusetts: Ion Exchange Treatment of Drinking Water Supplies. University of Massachusetts Extension, 2007.
Healthy Drinking Waters for Massachusetts: Iron and Manganese in Private Drinking Water Wells. University of Massachusetts Extension, 2007.
Healthy Drinking Waters for Rhode Islanders. Contribution number 3969, The College of the Environment and Life Sciences, University of Rhode Island. www.uri.edu/ce/wq/has/PDFs/Radon.pdf
Healthy Drinking Waters for Rhode Islanders: Ion Exchange Treatment of Drinking Water Supplies. Private Wells Series April 2003, Rhode Island Department of Health and the University of Rhode Island Cooperative Extension Water Quality Program.
Herman, G.M. and G.D. Jennings. 1996. Home Drinking Water Treatment Systems. North Carolina Cooperative Extension Service, Publication Number: HE-419. Available online at: www.bae.ncsu.edu/programs/extension/publicat/wqwm/he419.html.
Home Water Treatment Using Activated Carbon, WQ-13. Cooperative Extension Service, Purdue University, Lafayette, ID.
Ion Exchange Treatment of Drinking Water, WD-DWGB-2-12. Environmental Fact Sheet. New Hampshire Department of Environmental Services, Concord, NH, 2009. Available online at: http://des.nh.gov/organization/commissioner/pip/factsheets/dwgb/documents/dwgb-2-12.pdf
Jennings, G.D. and R.E. Sneed. 1996. Questions to Ask when Purchasing Water Treatment Equipment. Publication Number: AG 473-3, North Carolina Cooperative Extension Service. Available online at: www.bae.ncsu.edu/programs/extension/publicat/wqwm/ag473_3.html.
M. Kamrin, N. Hayden, B. Christian, D. Bennack and F. D'Itri. Reverse Osmosis for Home Treatment of Drinking Water, WQ-14, 3/91. Cooperative Extension Service, Purdue University, Lafayette, IN.
Manganese Greensand is Capable of Reducing Iron, Manganese and Hydrogen Sulfide from Water Through Oxidation and Filtration. Clack Corporation, Windsor, WI ().
Parrott, K. and B. Ross, J. Woodard. 2009. Household Water Treatment. Virginia Cooperative Extension, College of Agriculture and Life Sciences, Virginia Polytechnic Institute, Blacksburg and State University, Petersburg, VA.
Parrotta, M.J. and R. Bekdash. 1998. UV disinfection of small groundwater supplies. Journal AWWA. 90(2):71-81
Private Drinking Water in Connecticut: Ion Exchange Treatment of Private Drinking Water Systems. Publication No. 10. State of Connecticut Department of Public Health Environmental Health Section, Private Well Program, Hartford, CT, 2009. www.ct.gov/dph/lib/dph/environmental_health/pdf/ion_Exchange_Treatment_of_PDWS.pdf.
Questions to Ask When Purchasing Water Treatment Equipment . Publication 356-480 Virginia Cooperative Extension, College of Agriculture and Life Sciences, Virginia Polytechnic Institute and State University, 2009. Available online at: http://pubs.ext.vt.edu/356/356-480/356-480.pdf.
Saha, U., L. Sonon, D. Kissel, and R. Hitchcock. 2011. Total Coliform and Escherichia coli Bacteria in Georgia Private Wells: Relationships of Age and Depth of the Wells. In: Proceeding of the 2011 Land Grant and Sea Grant National Water Conference. January 31-February 1, 2011. Washington DC.
U.S. Environmental Protection Agency. 1996. Ultraviolet Light Disinfection Technology in Drinking Water Application—An Overview. Office of Water: Washington, D.C. EPA/811- R-96-002.
Ultraviolet Light Disinfection of Drinking Water: An Alternative to Chlorination. National Water Quality Program.
Ultraviolet Light Disinfection Technology in Drinking Water Application: An Overview, EPA- 811-R-96-002, National Service Center for Environmental Publications (NSCEP), USEPA, Office of Groundwater and Drinking Water, Washington, DC, 1996.
Understanding Home Water Treatment Systems, University of Missouri Extension, Columbia, MO. Available online at: extension.missouri.edu/publications/DisplayPrinterFriendlyPub.aspx?P=WQ104.
Wagenet, L. and A. Lemley. 2005. Water Treatment Notes: Guidelines for Purchasing Water Treatment Equipment. Cornell Cooperative Extension, College of Human Ecology. Available online at: http://waterquality.cce.cornell.edu/publications/CCEWQ-01-PurchaseWaterTrtEquip.pdf
Wagenet, L., K. Mancl, and M. Sailus. 1995. Home Water Treatment. Northeast Regional Agricultural Engineering Service, Cooperative Extension. NRAES-48. Ithaca, NY.
Water Treatment Using Carbon Filters: GAC Filter Information. Minnesota Department of Health, St. Paul, MN 55164, May 2008. Available online at: www.health.state.mn.us/divs/eh/hazardous/topics/gac.html.
Wellcare® Information for you about Well Water Treatment Options and Costs. Water Systems Council (WSC), Washington, DC, 2009. Available online at: www.watersystemscouncil.org/download/wellcare_information_sheets/well_water_testing_&_
treatment_information_sheets/DrinkingWaterTreatmentsandCostsFINAL.pdf
Wilson, A., K. Parrott, and B. Ross. 2009. Iron and Manganese in Household Water College of Agriculture and Life Sciences, Virginia Polytechnic Institute and State University.
Status and Revision History
Unpublished/Removed on Feb 19, 2009
Published with Major Revisions on Jul 19, 2011
Published with Full Review on Jul 25, 2014
Published with Full Review on Jun 20, 2018
Published with Full Review on Jun 22, 2023