Dennis W. Hancock, Extension Agronomist - Forage Crops, Crop & Soil Sciences Dept.
Glen H. Harris, Extension Agronomist - Soils and Fertilizer, Crop & Soil Sciences Dept.
Randy W. Franks, County Extension Coordinator, Wayne County
Steven P. Morgan, County Extension Coordinator, Harris County
T. Wade Green, County Extension Coordinator, Twiggs County
Exploring a Soil’s Characteristics
When designing a forage management system, it is important to understand the soil environment of the site. There are four levels or scales that must be considered when developing a management plan for a specific site: the geographic region, soil province, soil types, and site-specific conditions (Figure 1).
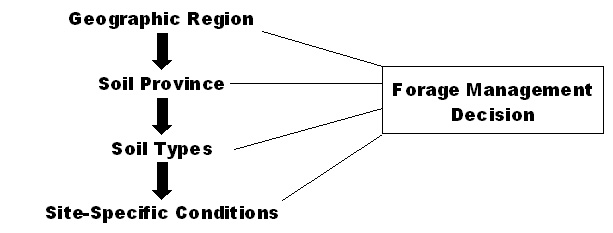 Figure 1
Figure 1. Forage management decisions are affected by soil characteristics on four basic scales.
Geographic Regions in Georgia
Essentially, Georgia can be thought of as having three main geographic regions: 1) the Limestone Valley/Mountains Region, 2) the Piedmont Region and 3) the Coastal Plain Region (Figure 2). Some forage management recommendations are based on these regional breakdowns. The two most notable examples of recommendations based on region are planting dates1 and variety recommendations2. These region-specific recommendations are primarily the result of climatic differences from one region to another rather than differences in soil characteristics.
Soil Provinces in Georgia
It is often helpful to understand soil differences within the geographic regions when refining forage management recommendations. Crop and soil scientists at the University of Georgia recognize six soil provinces in Georgia: 1) Limestone Valley, 2) Blue Ridge, 3) Southern Piedmont, 4) Sand Hills, 5) Southern Coastal Plain and 6) Atlantic Coast Flatwoods (Figure 3). These soil provinces differ from each other in many ways (e.g., texture, drainage, parent material, organic matter content, etc.). As a result, the forage system must accommodate these differences.
Limestone Valley
This province contains fertile upland soils and zones along streams that make excellent pastures. Good pastures can be produced on almost any land in the valleys. Steep and undulating terrain is mostly woodlands, but some areas can support pasture growth if care is taken to establish sod-forming permanent pastures. Soil erosion potential is high in the Limestone Valley and Blue Ridge soil provinces. When establishing or renovating pastures, establishment practices that minimize the risk of soil erosion (i.e., no-till or minimum till) should be employed. Cool-season perennials make excellent pasture in this province, but cold-hardy bermudagrasses are well-suited to hay lands and pastures in well-drained, sunny sites.
Though this area averages more than 52 inches of rainfall per year, dry weather frequently occurs in the spring, summer, and fall. Some use of drought-tolerant plants is recommended. (For more information about soils in the Limestone Valley, see the Crop and Soil Sciences Department Factsheet titled “Summary of Soil Test Results from Pastures and Hayfields Originating from the Limestone Valley Soil Province in Georgia between 1996 and 2007” at http://www.caes.uga.edu/commodities/fieldcrops/forages/soils/BR.html.)
Blue Ridge
The rich cove lands of this province are well-adapted to cool-season perennial pasture and hay production. Cold-hardy bermudagrasses can be used successfully for hay lands and summer grazing in this area, but are rare because of terrain and drainage issues. Winter annual pastures can be planted on any of the cultivated soils of this province. However, the upland soils have better drainage and are better suited to winter annual pasture. This area receives abundant rainfall (more than 65 inches per year in most areas). (For more information about soils in the Blue Ridge soil province, see the Crop and Soil Sciences Department Factsheet titled “Summary of Soil Test Results from Pastures and Hayfields Originating from the Blue Ridge Soil Province in Georgia between 1996 and 2007” at http://www.caes.uga.edu/commodities/fieldcrops/forages/soils/BR.html.)
Southern Piedmont
The Piedmont region of Georgia contains one large soil province, the Southern Piedmont. This is not to say, however, that areas within the Southern Piedmont are the same. Quite the contrary is true. In fact, the Southern Piedmont is difficult to characterize, as its soils are quite variable.
The Southern Piedmont contains more of the state’s forage-based livestock enterprises than any other soil province. Pastures in this region contain mixtures of cool-season and warm-season perennials, while hay lands are predominantly bermudagrass. Though there are exceptions, the lower Piedmont is generally considered the southern edge of adaptation for tall fescue and the northern edge of adaptation for bahiagrass. As a result, pastures in the lower Piedmont often contain significant amounts of bahiagrass, bermudagrass and tall fescue.
The best land for pastures is along the streams and river bottoms of this area. These low, moist zones are excellent for summer pastures, if adequately drained. Many parts of the Piedmont were extensively row cropped in the 19th and early 20th centuries. Severe soil erosion during this era resulted in the loss of much of the topsoil throughout this area. Though the upland soils still provide good spring and fall grazing, periodic droughts during the spring, summer and/or fall severely limit forage production in this area. Drought-tolerant plants should be used on the uplands for summer grazing. (For more information about soils in the Southern Piedmont, see the Crop and Soil Sciences Department Factsheet titled “Summary of Soil Test Results from Pastures and Hayfields Originating from the Southern Piedmont Soil Province in Georgia between 1996 and 2007” at http://www.caes.uga.edu/commodities/fieldcrops/forages/soils/SP.html.)
Sand Hills
Soil in the Sand Hills province is quite variable, and, as the name suggests, is characterized by sandy soils and undulating terrain. The Sand Hills province is located around the Fall Line (where the Piedmont transitions to the Coastal Plain).
Land that produces row crops in this area will provide acceptable forage yields. Some of the better areas will produce winter and summer annual pastures. However, because many of these soils are quite prone to drought, hybrid bermudagrasses that develop deep-root systems should be used for hay and grazing in this area. (For more information about soils in the Sand Hills, see the Crop and Soil Sciences Department Factsheet titled “Summary of Soil Test Results from Pastures and Hayfields Originating from the Sand Hills Soil Province in Georgia between 1996 and 2007” at http://www.caes.uga.edu/commodities/fieldcrops/forages/soils/SH.html.)
Southern Coastal Plain
Just south of the Sand Hills, the terrain in the upper sections of the Southern Coastal Plain becomes less rolling. Soils in this soil province are typically heavier and more fertile than the soils in the Sand Hills and Atlantic Coast Flatwoods. The best soils are in moist zones along streams. However, productive pastures can occur on better upland sites. Winter annual pastures often do best on upland soils in this area.
The Southern Coastal Plain is the second-largest soil province in Georgia and is home to the state’s official soil, the Tifton soil series. The Tifton soil series is the predominant soil series in the Southern Coastal Plain, occupying more than 75 percent of the lower and eastern part of this soil province. (For more information about soils in the Southern Coastal Plain, see the Crop and Soil Sciences Department Factsheet titled “Summary of Soil Test Results from Pastures and Hayfields Originating from the Southern Coastal Plain Soil Province in Georgia between 1996 and 2007” at http://www.caes.uga.edu/commodities/fieldcrops/forages/soils/SCP.html.)
Atlantic Coast Flatwoods
Flatwoods soils in Georgia are often poorly drained, with the water table periodically (usually in the winter) reaching within a few inches of the soil surface. Soils in this area also commonly contain organic hardpans. As a result, the best pasture soils are on good uplands and well-drained lowlands. Most of the uplands can produce winter annual pasture and perennial summer pasture. Closer to the Atlantic Coast, the soils are predominately poorly-drained and may not be suitable for pasture use. In the Flatwoods soils, bahiagrass swards generally will persist better than bermudagrass, unless the site is well-drained. (For more information about soils in the Atlantic Coast Flatwoods, see the Crop and Soil Sciences Department Factsheet titled “Summary of Soil Test Results from Pastures and Hayfields Originating from the Atlantic Coast Flatwoods Soil Province in Georgia between 1996 and 2007” at http://www.caes.uga.edu/commodities/fieldcrops/forages/soils/ACF.html.)
Soil Types in Georgia
There are literally hundreds of soil types in Georgia. It is not uncommon for a single pasture or hay field to contain several different soil types. Each soil type has its own characteristics. The easiest way to determine what soil types are on a given farm is to examine the soil survey (Figure 4).
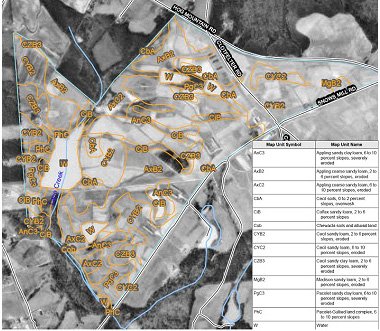 Figure 4.
Figure 4. Example of a soil survey map available from the USDA NRCS's Web Soil Survey ().
Most areas of Georgia have been surveyed by soil scientists from the USDA Natural Resources Conservation Service (NRCS). These soil surveys are published by the USDA NRCS either as surveys of single counties or combinations of two or three counties. Though not all counties have a modern soil survey available, NRCS soil scientists are working hard to provide statewide coverage. The status of soil survey work in Georgia can be found at .
Soil survey information is a powerful tool. In addition to outlining generalized physical and chemical properties of the soil types of interest, it can give relative estimates of crop performance. The soil survey even provides estimates of hay yields on a soil type and the number of animal units a particular soil type can carry.
Hard copies of the soil survey (assuming the survey for the area is complete) can be obtained from the local NRCS office, Conservation District, or library. Fortunately, the soil surveys of most counties in Georgia have been digitized and are available online via the USDA NRCS’s Web Soil Survey (). Tutorials and guides on how to use the Web Soil Survey are also available on their website.
Site-Specific Conditions
On every farm, there will be variation that cannot merely be explained by differences in soil type. These differences will often be substantial between fields, and conditions are often highly variable within a field. The variability may be the result of natural differences in soil formation, water-holding capacity, soil organic matter, slope or other factors. However, the most common contributor to differences between fields or areas within a field is historical management (e.g., pastures vs. hayfields, historical applications of nutrients, areas in a pasture where animals congregate versus areas where animals spend little time, etc.).
Soil conditions may be variable enough within a field that it warrants the identification of specific areas that are managed separately from other areas. Such “site-specific management” has been enabled by “precision ag” tools such as GPS, GIS, variable-rate applicators, etc3. These techniques are often more expensive than traditional, uniform management systems. As a result, site-specific management is usually not cost-effective, except for the most intensively-managed forage systems.
Most soil conditions are difficult or cost-prohibitive to change (e.g., soil water-holding capacity, organic matter, slope, etc.). However, the fertility of the soil is easier to improve. The first step in improving soil fertility is to take a soil test (see “Sampling the Soil in Pasture and Hayfields” below). Soil samples submitted through your county extension agent will be analyzed at the University of Georgia Agricultural and Environmental Services Laboratories’ Soil Lab. Lime and fertilizer recommendations will be made based on those soil test results.
Sampling the Soil in Pasture and Hayfields
 Figure 5.
Figure 5. Urine and dung patches should be avoided when sampling soils. The prevalence of these hummocks is a common indicator of nutrient deficiencies in other areas of the pasture.
A soil test is the best tool for assessing soil fertility. Soil testing is a chemical analysis that reveals any soil fertility issues that may be limiting production4. The soil sample analysis provides a guideline for the amount of lime or fertilizer needed to correct deficiencies or imbalances in soil pH or available nutrients. These amounts are determined by the specific needs of the crop being grown. Furthermore, soil test recommendations from the Cooperative Extension office are based on decades of scientific studies. Thus, by regularly testing soil and following the recommendations, soil fertility can be maintained at levels that result in optimum productivity of the pasture or hayfield.
The key to soil testing is to ensure that the sample is representative of the area of interest. At the very least, each field should be sampled separately. Soil pH and some nutrients will often vary with soil type. Fields with substantially different soil types should be sampled separately within major soil types.
When sampling pastures, be sure to avoid areas around water sources, shade, mineral feeders, where hay has been fed, or any other area where animals may have congregated and created a nutrient buildup. It is also important to avoid sampling in areas immediately surrounding urine or dung patches (Figure 5). In general, soil samples should be obtained from pastures every three years and from hayfields each year. More information on how to take a representative soil sample can be found in the Cooperative Extension Leaflet 99 titled “Soil Testing”.
The Importance and Role of Specific Nutrients and Soil Amendments
As with all crops, forages must be provided an ample supply of available nutrients. Maintaining optimum soil fertility is critically important for ensuring good establishment, persistence, winter hardiness, pest resistance, drought tolerance, sufficient forage quality, adequate yields, and economic returns. If any nutrient is deficient, problems in one or all of these areas can occur. Thus, it is critical that a good soil fertility program be the basis of any forage management system. This section presents factors that affect the availability of the nutrients in soil, briefly conveys the importance of several essential elements and identifies the most common sources of individual nutrients.
Soil pH
Soil pH measures soil acidity. Most forage crops grow best when the soil pH is 6.0 – 6.5. However, some legume species require a slightly higher soil pH (e.g., alfalfa requires a pH of 6.5 – 7.0). When soils are too acid (pH is too low), crop growth will be reduced. On the other hand, soils can become too basic (pH is too high) when too much lime is applied. Though this can also have a detrimental effect on plant growth, high soil pH values (> 7.0) are rare in Georgia.
When soil pH is kept at the level appropriate for the forage crop(s) being grown, the nutrients stored in the soil will be most freely available to the plant. This increases the plant's ability to efficiently use fertilizer and nutrients already in the soil. Proper soil pH also prevents high concentrations of toxic elements (e.g., aluminum) that can injure root tips and prevent proper rooting. Maintaining the appropriate soil pH also promotes desirable bacterial activity in the soil.
Most Georgia soils are acidic or will naturally become more acidic over time. The addition of ammoniacal forms of nitrogen fertilizer (e.g., ammonium sulfate, urea, UAN solutions, ammonium nitrate, etc.) can accelerate soil acidification. To correct low soil pH, the soil acidity must be neutralized. Lime supplies carbonate ions that neutralize soil acidity (increase soil pH). Agricultural lime is the most common product used to raise soil pH values, though other products (e.g., wood ash, marl, basic slag, egg shells, etc.) can also be used.
Liming agents differ in the amount of calcium and magnesium they contain. Both calcitic and dolomitic limestone contain calcium. However, dolomitic limestone also contains magnesium and should be used (if possible) to maintain sufficient soil magnesium levels. If magnesium is present in adequate levels, then calcitic limestone can be used.
One reason for maintaining a rather neutral soil pH is that it prevents aluminum (Al) from becoming soluble in the soil. When the pH drops, Al becomes dissolved in the soil moisture. Soluble Al is toxic to plants and drastically inhibits root growth. The addition of lime raises the soil pH, and the Al returns to a solid form.
Unfortunately, it is difficult for lime to quickly infiltrate deep into the soil profile. As a result, the soil surface may be neutral while the subsoil is very acidic. In this situation, the addition of gypsum (CaSO4) may be helpful for some crops. Although gypsum does not alter the soil pH, it can infiltrate the soil profile and reduce the toxicity of soluble Al. For example, research with alfalfa has shown significant yield increases in response to gypsum application on some soils with acidic subsoils. A subsoil sample (soil from deeper than 15 inches) must be tested to determine whether gypsum is needed.
Soil Organic Matter
Soil organic matter (OM) plays a critically important role in the biological, chemical, and physical characteristics of the soil. Soil OM supports soil microbes that are critical to making some essential nutrients available to the plant. Soil OM is also important in supporting populations of nitrogen-fixing bacteria that infect nodules on legume roots. The acidifying effects of ammonium fertilizers can be slowed by sufficient levels of soil OM. Soil OM also increases the ability of a soil to be well-drained while at the same time hold sufficient water to promote plant growth. In many of Georgia’s heavy clay soils, high levels of soil OM helps to prevent soil compaction.
Decaying roots, crop residue and animal dung provide the primary source of OM in the soils of pasture and haylands. To retain this OM, tillage operations should be kept to a minimum. Excessive tillage during seedbed preparations, use of aeration equipment, treading damage and other soil disturbances may decrease soil OM levels.
Essential Nutrients
Sixteen chemical elements are essential for normal plant growth and reproduction (Table 1)5. Some of these are non-mineral nutrients (e.g., hydrogen, carbon, oxygen, etc.) that are freely available to all plants, with very rare exceptions. However, several mineral nutrients may need to be supplemented.
| Table 1. The 16 nutrients that are essential for normal plant growth and reproduction. |
| Groups |
Essential Nutrients |
| Non-Mineral |
1. Carbon (C)
2. Hydrogen (H)
3. Oxygen (O) |
| Macronutrients |
| Primary |
4. Nitrogen (N)
5. Phosphorus(P)
6. Potassium (K) |
| Secondary |
7. Calcium (Ca)
8. Magnesium (Mg)
9. Sulfur (S) |
| Micronutrients |
10. Boron (B)
11. Chlorine (Cl)
12. Copper (Cu)
13. Iron (Fe)
14. Manganese (Mn)
15.Molybdenum (Mo)
16. Zinc (Zn) |
Essential nutrients are generally grouped into two categories, macronutrients and micronutrients, based on the concentration of the nutrients found in the plant. The nutrients required in the largest quantities are called macronutrients and are further grouped into primary and secondary nutrients. Primary nutrients are mineral elements that are needed in the highest concentration and that most frequently need to be supplemented. Primary nutrients include nitrogen (N), phosphorus (P) and potassium (K). Secondary nutrients (calcium (Ca), magnesium (Mg), and sulfur (S)) are also needed in high concentrations, but are not as frequently deficient in most soils. Other nutrients are also essential, but are required in much smaller quantities. These micronutrients include boron (B), chlorine (Cl), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), and zinc (Zn).
Georgia soils often do not contain sufficient concentrations of primary macronutrients. Occasionally, secondary macronutrients and micronutrients are not available in the appropriate concentrations for proper plant growth and the addition of fertilizer (inorganic or organic) may be necessary to correct the imbalance. Sometimes, however, this lack of nutrient availability (e.g., micronutrient deficiencies) may be because the soil pH has become too low or too high. Even when a deficiency does exist, there are many cases where the addition of the fertilizer may cost more than the value of the increased plant performance and/or come with some environmental consequence. Thus, the use of soil test-based recommendations from the University of Georgia is critical to the appropriate use of fertilizer.
Nitrogen (N)
Nitrogen is necessary for rapid growth and high yields, and is an essential component of plant proteins. The amount of N fertilizer needed and the correct timing of applications varies with crops and how they are used (for grazing or hay). Application rates for N fertilizer will typically be higher for hay crops than in pastures that are grazed, because N is recycled via the urine and feces of grazing animals. Since the amount of N available from the soil is typically much less than the forage could utilize, N can be effectively used as a tool to increase or decrease forage productivity in pastures, as needed.
Nitrogen-deficient plants will be light green or slightly yellow, especially in the lower (older) leaves, and will be much less vigorous. In pastures, N deficiency is often exhibited by a great difference in growth and color between spots where animals have urinated and the surrounding areas.
Phosphorus (P)
Phosphorus is an essential plant element that plays a key role in many vital plant processes such as root development, reproduction, and energy transfer. Low soil levels of P can cause difficulties in establishing new pastures. This element does not readily leach from most soils, and one application per year is sufficient.
Phosphorus levels in most of Georgia’s soils are naturally low. For forage crops, however, P deficiencies are less frequent than deficiencies in other nutrients. Applications of animal manures have occurred routinely on many areas where forage is produced. As a result, these soils are usually high in P. However, P deficiencies are quite problematic when they occur. Stands that are deficient in P will be stunted, but may be relatively dark green. In grasses, the base of the tiller is often dark purple. In legumes, the leaves will be much smaller than normal and older leaves may be dark green or purple.
Potassium (K)
Potassium is second only to nitrogen in the concentration found in plants, and is essential for producing economical yields (especially when stress conditions occur). It is also critical to maintaining thick, persistent stands (see insert, “Potassium Fertility for Bermudagrass”). It affects plant vigor, disease resistance, forage quality, and winter survival. It is important to split K applications across two or more application times to prevent excess K uptake (described in detail in the “Timing and Method of Nutrient Applications” section of this publication). This is particularly important with alfalfa and bermudagrass stands that are harvested for hay.
Potassium Fertility for Bermudagrass
Each ton of bermudagrass hay will often contain the equivalent of more than 40 lbs. of K fertilizer (K2O). High-producing bermudagrass hayfields may yield well over 10 tons per acre. As a result of this high rate of nutrient removal, K deficiencies occur frequently in bermudagrass hayfields. Stands that are K deficient become less vigorous, less dense, more disease prone, and more apt to winterkill.
In most cases, K deficiency comes about slowly. Deficiency symptoms occur initially in the margins of lower leaves in the form of chlorosis (yellowing) followed by necrosis (death). In fact, a bermudagrass stand may be very old before it begins to exhibit severe stand thinning as a result of K deficiency. However, some varieties are more prone to K deficiency problems than others. For example, “Alicia” is very susceptible to leafspot diseases when K deficiency occurs.
Research has shown that stands can recover if given adequate K supplementation. One major reason for this is that K fertility is critical for healthy rhizomes, the underground stems that aid the spread of bermudagrass. Rhizome production is nearly 800 percent greater when K fertilization is adequate than when K is deficient.
Sulfur (S)
Sulfur is critical to protein formation, N-fixation in legumes, and maintaining root growth. Sulfur may become a limiting nutrient in plants that accumulate high levels of nitrogen in their tissues. In Georgia, the need for S varies considerably. Like N, the S in the soil is held and released from soil OM and it will leach out of the soil. Much of the S available to the plant results from atmospheric deposition of S that was released during the burning of fossil fuels (coal, gas, diesel, etc.). Another substantial source of S is animal waste (especially poultry litter). As the OM in the animal waste breaks down, an abundance of S is supplied to the plants.
Deficiencies do occasionally occur, especially on soils in the Coastal Plain Region that are deep sands and have lower OM. Drought or low pH can cause slow OM decomposition and can also be a factor in S deficiency, which is often confused with N deficiency since the two share symptoms of yellowing and stunted appearance. However, they can be differentiated based on where the symptoms occur on the plant. Sulfur is less mobile than N and deficiency symptoms tend to first appear in younger leaves, in contrast to N deficiency, which tends to appear first in older leaves.
Calcium (Ca)
Calcium is critical for several basic plant functions (cell growth, stress detection, signaling, cell division, etc.). Fortunately, Ca deficiencies are rare in Georgia, especially if the soil has been limed. Legumes accumulate higher levels of calcium than grasses.
Magnesium (Mg)
Magnesium is a critical element of chlorophyll, the green pigment in plants that enables photosynthesis. It is fairly common for Mg to be deficient in Georgia, especially on acid, sandy soils in the Coastal Plain region. Magnesium deficiency causes yellowing between the veins of the leaf and will be found first in the lower or older leaves of the plant. Dolomitic limestone can be used to increase soil Mg and reduce deficiencies.
Magnesium deficiency is often more problematic when the forage is growing quickly. The uptake of Mg by plant roots is sometimes slow, especially if K is high. In addition to the detrimental effects that Mg deficiency has on the plants, animals grazing forage that is low in Mg may develop low Mg levels in the blood. This causes hypomagnesemia (grass tetany), and these animals may stagger, collapse, convulse, and, if not given timely treatment (calcium-magnesium gluconate), can die.
Micronutrients
In general, forages in Georgia rarely need to be supplemented with micronutrients. Occasionally, studies have shown a yield increase as a result of fertilizing with micronutrients (usually a foliar spray). However, these applications are expensive and rarely cost-effective. Usually, micronutrient deficiencies are a symptom of a soil that is too acidic or basic.
There are two notable exceptions to this generalization: B and Mo. These nutrients are needed in small quantities by plants, but supplementing with B and Mo may occasionally be necessary for proper legume growth, as they play significant roles in nodule formation and N-fixation. For alfalfa, an annual application of B (three pounds B per acre) is recommended, and an application of Mo (eight ounces of sodium molybdate in 25 gallons of water per acre) should be made every other year. The need for B supplementation in grasses is rare.
Nutrient Sources
There are many materials (both organic and inorganic) that can be added to the soil that provide nutrients. Commercial fertilizers provide these nutrients in relatively concentrated forms. Animal wastes and other organic sources of nutrients will typically provide fewer nutrients per pound of product and be highly variable. Regardless of nutrient source, it is important to understand what and how many nutrients are being provided by the product (Table 2). Commercial fertilizers will have guaranteed analysis. However, animal wastes and other such materials need to be tested for nutrient content because of their variability from source to source. Additionally, the effect a nutrient source may have on soil pH should be considered.
| Table 2. Selected sources of nutrients that are commonly used in forage production. |
Nutrient Source |
N |
P2O5 |
K2O |
S |
Ca |
Mg |
Effect on Soil pH† |
Comments |
| —————— % —————— |
| Ammonium Nitrate |
34 |
— |
— |
— |
— |
— |
↓↓ |
Not as widely used as in the past. Usually not available in smaller markets. |
| Ammonium Sulfate |
21 |
— |
— |
24 |
— |
— |
↓↓↓↓ |
Very acidifying effect on soil. |
| UAN Solution |
28-32 |
— |
— |
— |
— |
— |
↓↓ |
Liquid nitrogen solution containing urea and ammonium nitrate. |
| Urea |
46 |
— |
— |
— |
— |
— |
↓↓ |
Prone to substantial volatilization losses. |
| Urea (Sulfur-coated) |
38 |
— |
— |
16 |
— |
— |
↓↓↓ |
Sulfur reduces volatility, but increases the negative effect on soil pH. |
| Diammonium Phosphate |
18 |
46 |
— |
— |
— |
— |
↓↓↓ |
Commonly called DAP. Used to provide P and part of N needs. |
| Monoammon. Phosphate |
11 |
48 |
— |
— |
1 |
— |
↓↓↓↓ |
Commonly called MAP. Also used to provide P and part of N needs. |
| Triple Superphosphate |
— |
46 |
— |
2 |
14 |
— |
None |
Usually used in blends with other fertilizers or when fertilizing legumes. |
| Muriate of Potash |
— |
— |
60 |
— |
— |
— |
None |
One of the most widely-used fertilizers in the world. Commonly used in blends. |
| Poultry Litter (Broiler) |
3 |
3 |
2 |
1 |
2 |
— |
↑ |
Highly variable. Only 50% of N is available. |
| Cattle Manure |
1.5 |
1.5 |
1.2 |
— |
1.1 |
0.3 |
↓ |
Data represent feedlot manure. Barn manure will be much lower in N. |
| Horse Manure |
1.0 |
0.7 |
1.8 |
|
|
|
|
Highly variable. Very little N will be available immediately. |
| Sulfate of Potash Magnesia |
— |
— |
21 |
23 |
— |
11 |
None |
Second most common form of K fertilizer. |
| Calcitic limestone |
— |
— |
— |
— |
36 |
— |
↑↑↑↑↑ |
A common, high-calcium type of ag lime in Georgia. |
| Dolomitic limestone |
— |
— |
— |
— |
24-30 |
6-12 |
↑↑↑↑↑ |
A common, magnesium-containing ag lime in Georgia |
| Gypsum |
— |
— |
— |
18 |
22 |
— |
None |
Naturally mined or a by-product of coal-burning power plants or the phosphate industry. Can improve soil structure and reduce soluble Al in acidic soils. |
| † Number and direction of the arrows indicate the relative effect that the nutrient source will have on soil pH (e.g., ↓ indicates slight decrease in soil pH, ↑↑↑↑↑ indicates substantial increase in soil pH). In the case of N—containing fertilizers, these relative indicators are corrected on the basis of their N content (i.e., lbs. of CaCO3 required to neutralize 1 lb. of N). |
Timing and Method of Nutrient Applications
Fertilizers and other nutrient sources are quite valuable and potent. As a result, they pose economic and environmental risks. Nutrients that end up in runoff, groundwater, or the air reduce economic efficiency and impair water and air quality. Consequently, it is important to apply these nutrients at a rate, at a time and in a way that maximizes the efficiency of their use and minimizes their environmental impact.
Forages differ in the amount of nutrients required and the time of year in which the nutrients are needed. These crop-specific recommendations are provided for each forage crop in the Soil Test Handbook for Georgia (). The crop-specific recommendations are also printed on every soil test report, along with any additional comments or recommendations by the county extension agent. More information and up-to-date soil fertility recommendations for forages can be found in the Soil Test Handbook or on the “Fertilization Guidelines” page of the Georgia Forages Web site ().
In this section, recommended application timing and methods will be presented for lime and the three most commonly applied nutrients: N, P, and K. This is presented from the perspective of common forage production constraints (i.e., predominantly permanent sod/no-tillage systems, erodible soils, etc.) and may not be applicable to other production systems.
Applying Lime
In general, lime applications can be made anytime during the year. However, consideration must be given to the fact that lime may take several months (sometimes more than a year) to be fully effective. If changes or new plantings are planned and the soil test indicates lime is needed, lime applications should be made at least eight months prior to new plantings or other such renovations. High-quality liming materials are available that act faster than traditional “ag lime,” but they are generally less cost-effective for forage production scenarios. If the soil is to be plowed and/or prepared with conventional tillage, the lime application should be split so that half is applied prior to plowing and the other half applied during final seedbed preparation stages.
Research has shown that the infiltration of lime into the top two feet of the soil profile is generally better in no-till and permanent sods than in soils that are routinely turned (plowed and prepared with conventional tillage). One of the major reasons for this is that larger pores exist in undisturbed soil than in tilled soil as the result of natural soil particle aggregations, channels that form around decaying roots, and activity by earthworms and other soil biota. Proper soil pH is critical to maintaining these large pores. Thus, it is important that lime applications are made whenever regular soil tests indicate a need.
When lime is needed on pastures or hayfields, ensure that the soil is dry enough to support heavy equipment traffic. Ruts or compaction made in permanent sods during lime application will be especially difficult to manage around, expensive to remediate, and make harvesting or mowing more difficult.
Applying Nitrogen
Nitrogen fertilizers generally contain some percentage of ammoniacal (NH2, NH3 or NH4) or nitrate (NO3) forms of N or both. Organic N, such as in animal manures, generally contains N in the form of urea, ammonium (NH4), uric acid, amino acids, and additional N forms that become available to the plant over time. For example, Table 3 shows the differences in N content and form that exists between the manure from selected livestock species.
| Table 3. Approximate amount of N and N forms in the manure of selected livestock species.† |
Animal Species |
Total N |
Amino Acid |
Urea |
NH4 |
Uric Acid |
Other |
| (%) |
————— (% of Total N) ————— |
| Poultry |
3.0 |
27 |
4 |
8 |
61 |
1 |
| Beef |
1.5 |
20 |
35 |
<0.5 |
— |
44 |
| Dairy |
1.0 |
23 |
28 |
<0.5 |
— |
49 |
| Horse |
1.0 |
24 |
25 |
<1.0 |
— |
49 |
| Swine |
1.0 |
27 |
51 |
<0.5 |
— |
22 |
| Sheep |
2.0 |
21 |
34 |
<1.5 |
— |
43 |
| † Adapted from Havlin et al., 1999. “Soil Fertility and Fertilizers: An Introduction to Nutrient Management” pg. 137 6th ed. Prentice Hall, Inc. |
Plants can take up and use N either as ammonium or nitrate. Unfortunately, not all the N that is applied to the soil will make it into the soil, be made available to the plant, be taken up by the plant or be held by the soil. This lost N can pose significant economic and/or environmental risks. This section describes the mechanisms of N loss, presents conditions that make the losses worse and lists practices that can reduce the risk of loss (Table 4).
Volatilization
When urea (either as a fertilizer or in animal manures), uric acid (found in poultry litter), or other organic N sources are applied, these N forms may be enzymatically converted to ammonia (NH3). Ammonia is typically then converted to ammonium (NH4). Ammonium that is not taken up by the plant can be absorbed and held by the soil or tied up in less available forms (immobilization or ammonium fixation) or converted to nitrate by soil bacteria (mineralization and nitrification). However, ammonia is a volatile gas that can escape to the atmosphere before the N source makes it into the soil and is converted to ammonium.
Fertilizers such as ammonium sulfate, ammonium nitrate and other nitrate-based sources of N do not have ammonia as a direct intermediary step. Therefore, these fertilizers do not result in ammonia loss. Urea-ammonium nitrate (UAN) solutions may have some volatilization loss, but since half its N is in the form of ammonium nitrate, its volatilization losses will typically be half that of urea alone. Still, applications of UAN or other urea or organic N sources should be made in a way that minimizes the risk of volatilization (Table 4).
| Table 4. Factors affecting the four key mechanisms of N loss and the practices that can decrease the risk. |
| Volatilization |
| Factors that Increase the Risk of N Loss |
Practices that Decrease the Risk of N Loss |
- Hot, humid conditions
- High wind speeds
- Soils that are sandy and/or low in buffering capacity
- Use of N sources that contain urea (fertilizers and animal manures) or uric acid (primarily poultry litter)
- Small droplet size when using liquid N (UAN soln.)
|
- Use ammonium nitrate or other nitrate sources as the N fertilizer to eliminate the risk.?
- Time the N application to occur before a rainfall event (> 0.50 in.) or irrigation.
- Avoid using broadcast spray nozzles when applying liquid N products. Use dribble or flood nozzles to obtain larger droplet sizes.
- Split N applications between harvests (grazings or hayings), applying N prior to each growth period.
- Avoid applying urea-based products when it is excessively hot and humid.
- If urea-based fertilizers are used, volatility may be reduced by using a product treated with either the urease-inhibitor NBPT (N-(n-butyl) thiophosphoric triamide, trade name Agrotain™) or a controlled-release polymer (ESN®) technology.?
|
| Leaching |
| Factors that Increase the Risk of N Loss |
Practices that Decrease the Risk of N Loss |
- High N fertilization rates at one time
- Slow rate of N uptake by the plant
- Overgrazed pastures
- Well-drained soils that regularly receive soaking rains or are over-irrigated
|
- Split N applications between harvests (grazings or hayings), applying N prior to each growth period.
- Use slow-release N fertilizer or organic N products.
- Promote good root development by rotational grazing and preventing overgrazing.
|
| Runoff |
| Factors that Increase the Risk of N Loss |
Practices that Decrease the Risk of N Loss |
- Overgrazed pastures or very short forage canopy
- Sloped areas within a field
- Poorly drained or water-logged soils
|
- Avoid overgrazing pastures.
- Minimize the N rate applied to sloping areas within a field.
- Split N applications between harvests (grazings or hayings), applying N prior to each growth period.
- Use a highly-soluble N fertilizer that enables the N to quickly dissolve and infiltrate into the soil before high rainfall intensities result in runoff or erosion (Table 5).
- Avoid applying N to saturated soils.
|
| Denitrification |
| Factors that Increase the Risk of N Loss |
Practices that Decrease the Risk of N Loss |
- Poorly drained or water-logged soils
- High N fertilization rates
- High OM content in soils
|
- Split N applications between harvests (grazings or hayings), applying N prior to each growth period.
- Minimize the N rate applied to poorly drained or saturated soils.
- Use slow-release N fertilizer or organic N products.
|
? This practice eliminates volatilization loss. However, availability of ammonium nitrate and other nitrate sources may be low in many areas or cost prohibitive.
? Research results in row crops and (to a lesser degree) in forage crops indicate that both the urease-inhibitor NBPT (N-(n-butyl) thiophosphoric triamide, trade name Agrotain™) and a controlled-release polymer (ESN®) have the potential to reduce the loss of N to volatilization. Other such products have failed to demonstrably reduce N volatility or have not been adequately evaluated. Even when effective at reducing N lost to volatilization, it is still not clear that these fertilizer treatments will be of economic benefit to forage producers. |
Volatilization loss is made worse by hot, humid and windy conditions. Furthermore, forage crop residue and thatch contain more urease and ureolytic microbes than the underlying soil. As a result, 10 to 25 percent of the total N applied is usually lost to volatilization (ammonia gas) when urea or animal wastes are applied to forage lands. Under the most severe conditions, volatilization losses of more than 45 percent of the total N applied (as urea) have been observed in Georgia.
Volatilization losses are minimized when N applications are quickly followed by rainfall events (> 0.50 inches). Unfortunately, rainfall events are quite random during the growing season, especially during the summer months. Often, little or no rain will occur when one would prefer to apply fertilizer.
Runoff
In contrast, heavy rains may occur and cause significant runoff. This can result in environmentally significant losses of N if heavy rains occur before the N has percolated into the soil. For producers who use poultry litter as the primary source of N, runoff poses a significant risk of contaminating surface waters. Though N runoff can occur with commercial fertilizers, University of Georgia researchers have shown that N in runoff was substantially greater from pasture areas treated with poultry litter than from pasture areas treated with an inorganic (highly soluble) commercial fertilizer (Table 5). Management practices that minimize runoff are presented in Table 4.
| Table 5. Nutrients in runoff from both an area fertilized with broiler litter and a commercial fertilizer containing nutrients equivalent to the broiler litter.* |
Nutrient Source |
Ammonium N |
Dissolved
Reactive P |
Total P |
| |
(lbs. NH4-N/acre) |
----- (lbs. P/acre) ----- |
| Broiler Litter |
0.70 |
0.52 |
0.66 |
| Equivalent Commercial Fertilizer |
0.28 |
1.62 |
1.96 |
| * Average of two runs (late April and late May 2002) of a rainfall simulation on a mixed tall fescue-bermudagrass pasture at the Central Georgia Research and Education Center in Eatonton. These simulations were designed to examine the effect of a fairly heavy rainfall event (one inch of rain over 30 minutes). |
Leaching
Another potential mechanism of N loss is nitrate leaching. When high N rates are applied and/or high rainfall rates occur, the plants may not be able to take up nitrate fast enough. As moisture percolates down through the soil profile, some nitrate may be leached away in it. Ultimately, the nitrate may be taken below the rooting depth and may end up in the groundwater. Although high nitrate content in water systems poses a substantial risk to water quality, nitrate leaching from properly managed pastures and hayfields is generally expected to be fairly low. Management practices that minimize the risk of leaching losses are presented in Table 4.
Denitrification
One final mechanism of N loss is denitrification. Denitrification is the process by which nitrate is transformed by soil bacteria into nitrogen oxides (NO and N2O) and nitrogen gas (N2). This process occurs when soils become waterlogged and low in oxygen. Thus, N applications to poorly drained or saturated soils should be minimized or avoided. Denitrification losses are also generally expected to be quite low. However, the use of recommended management practices presented in Table 4 will ensure that denitrification losses are insignificant.
Splitting Applications
Applying large rates of N at one time results in inefficient N use because of these losses. By dividing the total recommended N rate by the number of anticipated harvests (grazings or hay cuttings) and applying these smaller amounts prior to each growth period, the economic and environmental risk of N loss will be greatly reduced (Table 4). For example, long-term hay production studies in Tifton have shown that N is used much more efficiently by bermudagrass (25 to 30 percent increase in the lbs of forage produced per lb of N added) when N applications are split between the expected harvests and adjustments are made for poor growing conditions (especially drought) rather than provided all at green-up. Further, this long-term study also demonstrated that splitting N applications can increase hybrid bermudagrass yields by 10 to 20 percent.
Applying Phosphorus
When P is applied to the soil, the fertilizer dissolves into rainwater and/or soil moisture. It reacts very rapidly with soil particles, soil OM and Fe/Al oxides in the soil. Once this occurs, the P is tightly held in the soil and becomes much less soluble than the form in which the fertilizer was added. Consequently, P is not very mobile in the soil, and leaching losses are generally insignificant. Phosphorus is also not subject to gaseous losses.
Since P applied to the soil is relatively stable and generally available to the plant as it is needed, P fertilizer can be applied virtually any time during the year on forage crops. This flexibility in application timing allows the producer to purchase P fertilizer in “off-peak” times of the year (i.e., summer and fall) when demand for the product and spreading services is lower. One exception to this flexibility is that the recommended P rate should always be applied before planting annual crops or plantings of new perennial forages.
Producers should avoid spreading P fertilizer when the risk of runoff is high (November through March in Georgia). The loss of P in runoff is the primary way in which P is lost from soils. Phosphorus loss occurs when P-containing soil or organic matter particles are eroded away in runoff water and when runoff water dissolves P from surface-applied fertilizers and manures. Runoff that contains high P levels can end up in surface waters and lead to eutrophication, excessive algal growth and hypoxia. Thus, the presence of elevated P concentrations in the runoff from pastures, hayfields and associated livestock facilities are of concern. This runoff can occur regardless of the source of P being used, but when used at similar P rates, losses from commercial fertilizers can be much larger than losses from poultry litter (Table 5).
Therefore, producers should take precautions to ensure that P applications are made in ways that minimize the risk of runoff, especially when dealing with inorganic P sources (i.e., commercial fertilizer). Table 6 presents the factors that increase the risk of P loss in runoff and management practices that can reduce this risk.
| Table 6. Factors affecting P loss via runoff and practices that can decrease the risk of P loss. |
| Runoff |
| Factors that Increase the Risk of P Loss |
Practices that Decrease the Risk of P Loss |
- Overgrazed pastures or very short forage canopy
- Sloped areas within a field
- Poorly drained or water-logged soils
- Use of inorganic P sources
- Applying animal waste at a rate meant to meet the N needs of the crop (i.e., an excessive P rate)
|
- Avoid overgrazing pastures.
- Minimize the P rate applied to sloping areas within a field or to poorly-drained soils.
- Apply P during times of the year when heavy rains are less likely.
- Avoid applying P to saturated soils.
|
Applying Potassium (K)
In contrast to N and P, the environmental risk posed by K is very low. However, K is quite expensive and necessary for optimum forage production. As a result, K applications should be made in a way that maximizes the availability of K over the entire growing season.
Muriate of potash (KCl) is by far the most common K fertilizer, though other K fertilizers are occasionally used. Substantial amounts of K may also be found in animal wastes. However, if these products are applied at rates designed to supply recommended N and P levels, additional K may be needed on K-deficient soils.
When K comes in contact with water, it quickly dissolves and enters the soil. Potassium is a cation (has a positive charge) and is attracted to the soil (which carries a negative charge). As a result, the risk of K runoff is quite low. Furthermore, very little K remains dissolved in the soil water. Thus, losses of K to leaching are lower than losses of nitrate.
However, some soils in Georgia have a very low cation exchange capacity (CEC), which means they do not have much capacity for attracting cations (K, Ca, Mg, etc.) or making them available to the plant. Consequently, significant amounts of K can be lost to leaching in those soils. This problem is more common in the sandy soils in the Coastal Plain region and can be exacerbated by low soil pH.
Nonetheless, the biggest potential for inefficient use of K is a phenomenon called “luxury consumption.” Most plants (especially forage crops) will take up more K than is required for optimum growth. Thus, if relatively large rates of K are applied early in the growing season, forage crops will absorb excess K and reduce the amount available for later growth cycles.
Because of this potential for luxury consumption and (in some cases) K loss to leaching, it is recommended that K applications be split across two or more application times. This will lower the risk of luxury consumption and leaching, allowing K to be used more efficiently and be available throughout the growing season. This is particularly important for forage crops that are harvested for hay or silage.
Further Information
Cooperative Extension Leaflet 99 Bulletin Soil Testing.
Soil Test Handbook for Georgia ().
Cooperative Extension Bulletin 1330 Poultry Litter Application on Pastures and Hayfields.
Cooperative Extension Bulletin 1230 Best Management Practices for Storing and Applying Poultry Litter.
Cooperative Extension Bulletin 1245 Maximizing Poultry Manure Use Through Nutrient Management Planning.
NRCS’s Web Soil Survey ().
Summary
Georgia possesses diverse soil conditions and many forage production factors are influenced by this diversity. As a result, the soil environment of a given site must be considered when selecting forage species, determining fertilization strategies and planning forage utilization systems. This article guides forage producers through the process of exploring their soil’s characteristics and sampling the soil in pastures and hayfields for testing, and provides information about specific nutrients and soil amendments relative to forage production practices. Recommendations are also made on how to minimize the economic and environmental risks associated with the addition of nutrients to pasture and hayfields.
1 See Cooperative Extension Circular C-814: Planting Guide to Grasses and Legumes for Forage and Wildlife in Georgia.
2 See the Web site titled “Forage Species and Varieties Recommended for Use in Georgia” ().
3 Global Positioning Satellites (GPS) allow the precise positioning of points, lines or shapes (polygons) within a field or location. Geographic Information Systems (GIS) are record-keeping and management software programs that allow for the collection and management of date on these points, lines and shapes. Collectively, GPS and GIS have enabled the management of specific sites within a field.
4 Some essential nutrients (e.g., nitrogen, sulfur and boron) are not consistently held in the soil and are not analyzed in routine soil tests. Recommendations for these nutrients are made based on the results from many research trials.
5 Nutrients that are essential for plant growth share some similarity to nutrients essential for animal growth; however, there are some substantial differences. Some micronutrients, including selenium, chromium, cobalt, sodium and iodine, are not required by plants but must occur at critical levels in the animal’s diet. Deficiencies of these nutrients in the animal are often solved most economically by providing the animal with mineral supplements.
Status and Revision History
Published on Nov 17, 2008
Published with Full Review on Nov 08, 2011
Published with Full Review on Nov 30, 2014